Label: IN TRANSIT SKIN DEFENCE- avobenzone, octinoxate, octocrylene, oxybenzone cream
- NDC Code(s): 71278-006-01
- Packager: THIS WORKS PRODUCTS LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
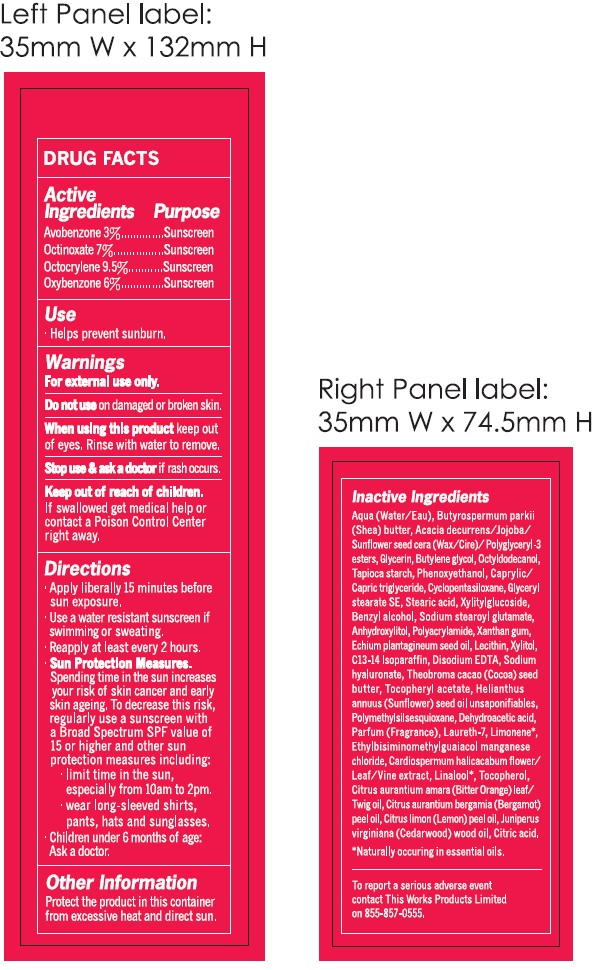
- DRUG FACTS
- Active Ingredients
- Use
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Spending time in the sun increases your risk of skin cancer and early skin ageing. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10am to2pm.
- wear long-sleeved shirts, pants, hats and sunglasses.
- Children under 6 months of age: Ask a doctor.
- Other Information
-
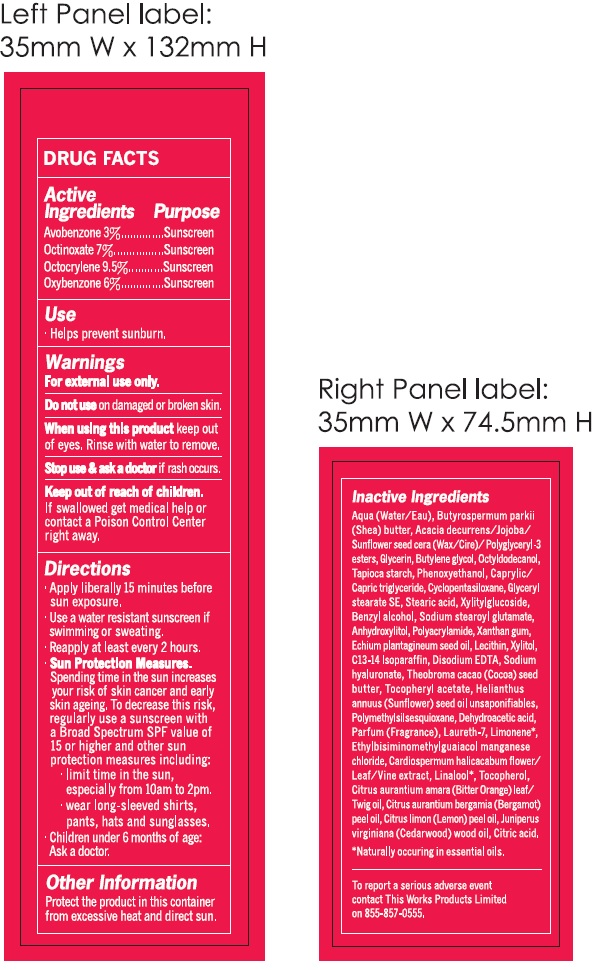
Inactive Ingredients
Aqua (Water/Eau), Butyrospermum parkii (Shea) butter, Acacia decurrens/Jojoba/Sunflower seed cera (Wax/Cire)/Polyglyceryl-3 esters, Glycerin, Butylene glycol, Octyldodecanol, Tapioca starch, Phenoxyethanol, Caprylic/Capric Triglyceride, Cyclopentasiloxane, Glyceryl stearate SE, Stearic acid, Xylitylglucoside, Benzyl alcohol, Sodium stearoyl glutamate, Anhydroxylitol, Polyacrylamide, Xanthan gum, Echium plantagineum seed oil, Lecithin, Xylitol, C13-14 Isoparaffin, Disodium EDTA, Sodium hyaluronate, Theobroma cacao (Cocoa) seed butter, Tocopheryl acetate, Helianthus annuus (Sunflower) seed oil unsaponifiables, Polymethylsilsesquioxane, Dehydroacetic acid, Parfum (Fragrance), Laureth-7, Limonene, Ethylbisiminomethylguaiacol manganese chloride, Cardiospermum halicacabum flower/Leaf/Vine extract, Linalool, Tocopherol, Citrus aurantium amara (Bitter Orange) leaf/Twig oil, Citrus aurantium bergamia (Bergamot) peel oil, Citrus limon (Lemon) peel oil, juniperus virginiana (Cedarwood) wood oil, Citric acid. *Naturally occuring i essential oils.
- QUESTIONS
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
IN TRANSIT SKIN DEFENCE
avobenzone, octinoxate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71278-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 95 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OCTYLDODECANOL (UNII: 461N1O614Y) STARCH, TAPIOCA (UNII: 24SC3U704I) PHENOXYETHANOL (UNII: HIE492ZZ3T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) STEARIC ACID (UNII: 4ELV7Z65AP) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) ANHYDROXYLITOL (UNII: 8XWR7NN42F) XANTHAN GUM (UNII: TTV12P4NEE) ECHIUM PLANTAGINEUM SEED OIL (UNII: PIB7XBU8XW) XYLITOL (UNII: VCQ006KQ1E) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) COCOA BUTTER (UNII: 512OYT1CRR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) DEHYDROACETIC ACID (UNII: 2KAG279R6R) LAURETH-7 (UNII: Z95S6G8201) LIMONENE, (+)- (UNII: GFD7C86Q1W) ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE (UNII: SM5YJ88LTU) CARDIOSPERMUM HALICACABUM FLOWERING TOP (UNII: MZP2508BRR) LINALOOL, (+/-)- (UNII: D81QY6I88E) TOCOPHEROL (UNII: R0ZB2556P8) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) BERGAMOT OIL (UNII: 39W1PKE3JI) LEMON OIL (UNII: I9GRO824LL) JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71278-006-01 1 in 1 CARTON 05/17/2017 1 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/17/2017 Labeler - THIS WORKS PRODUCTS LIMITED (211715561)