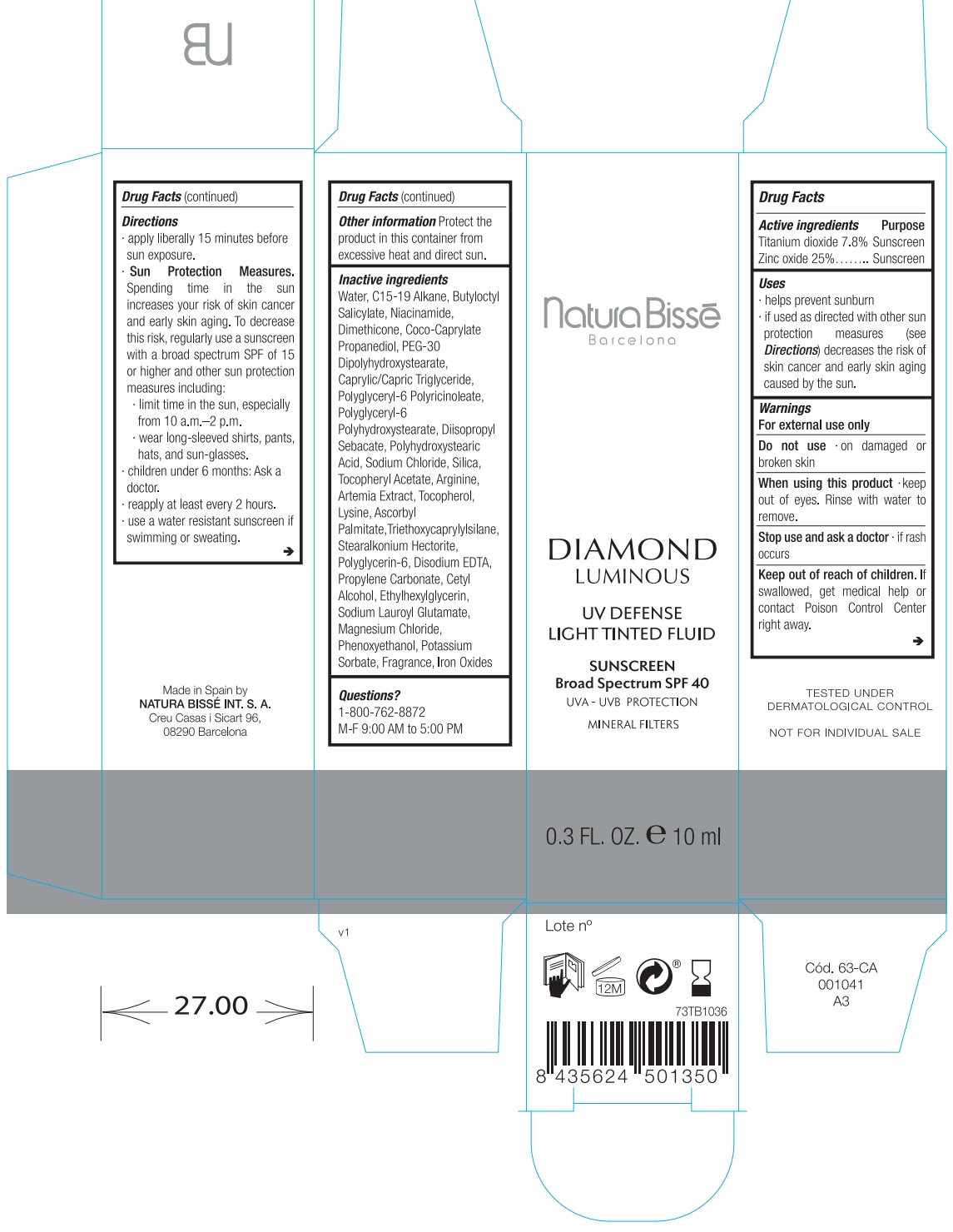
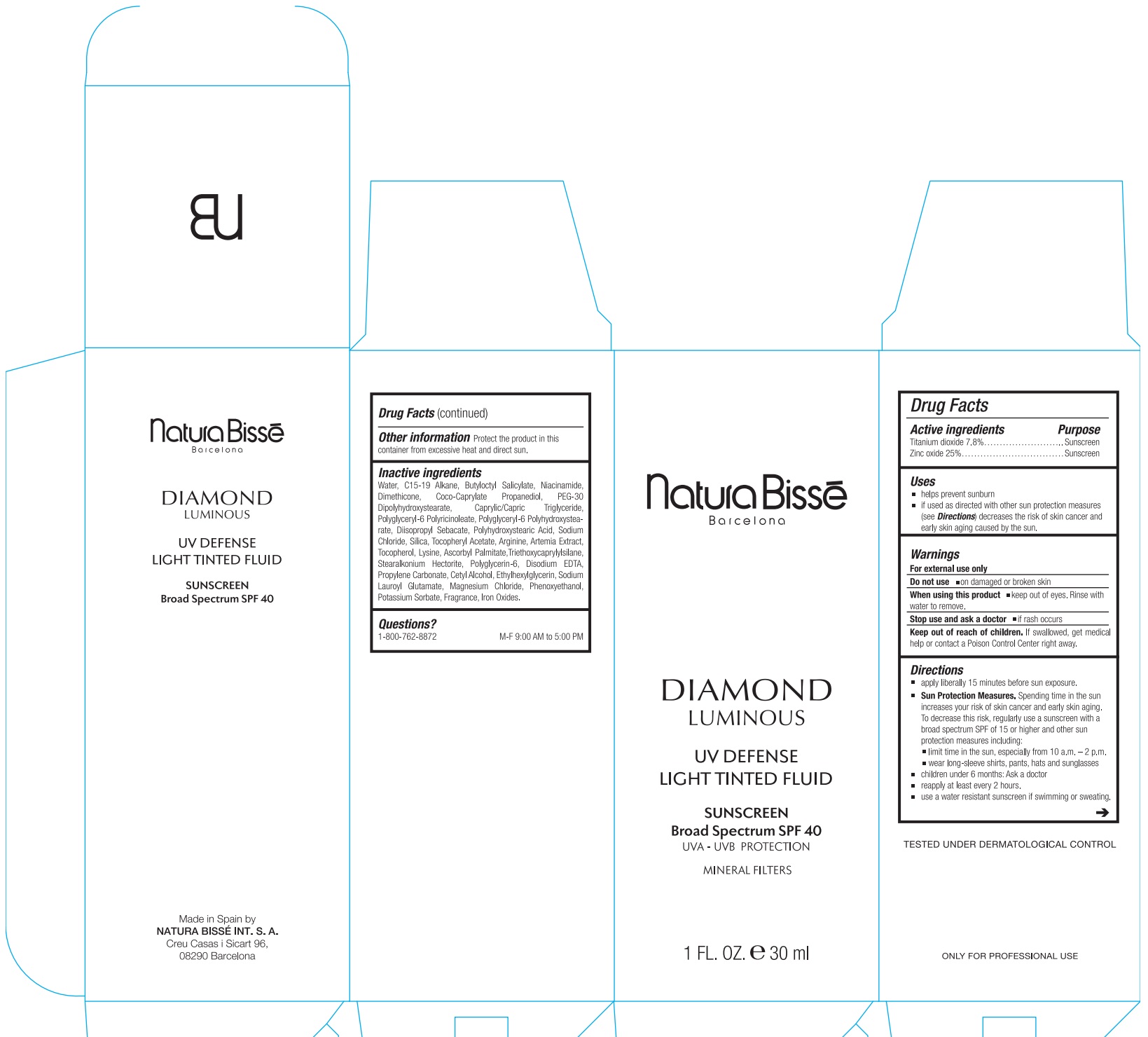
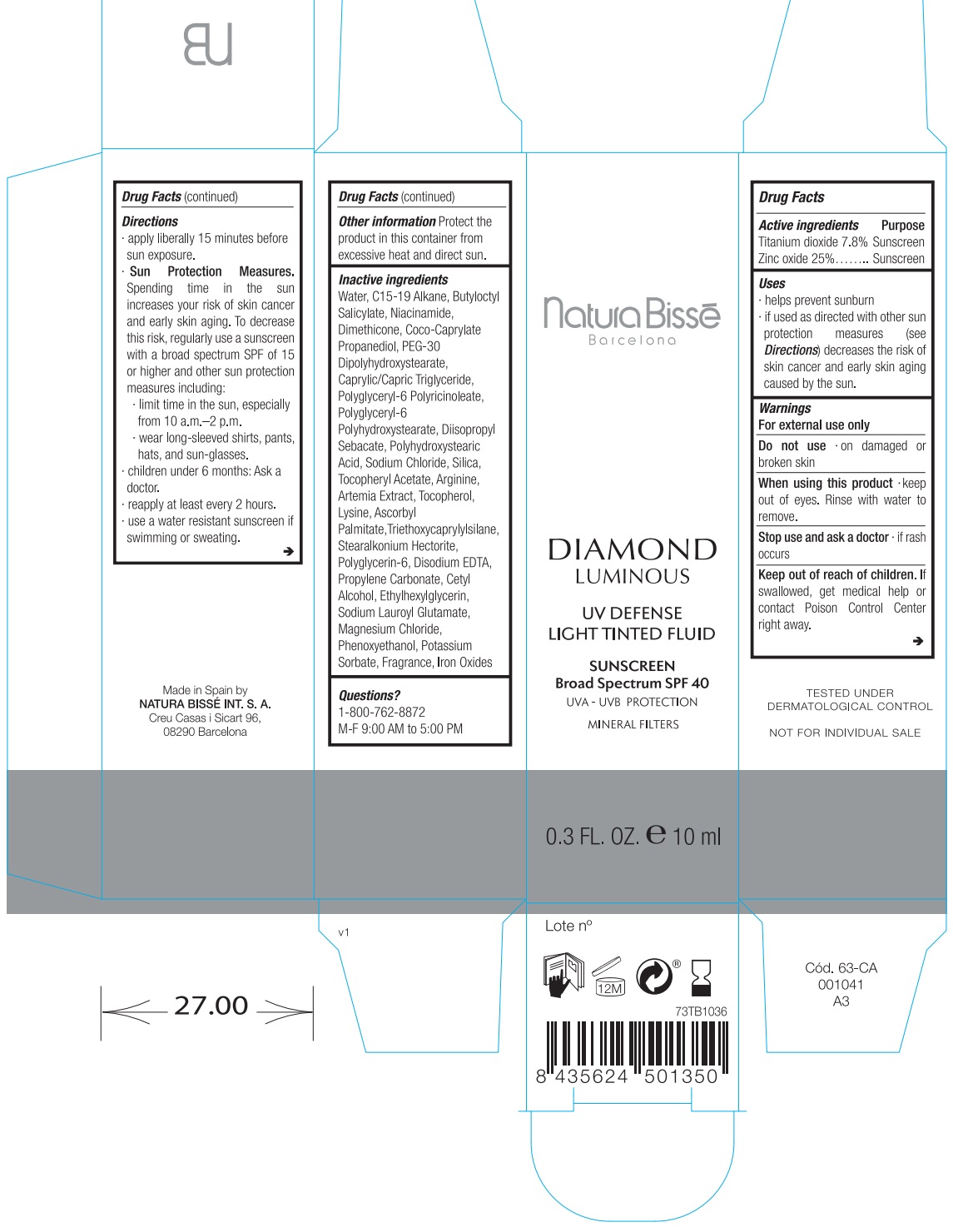
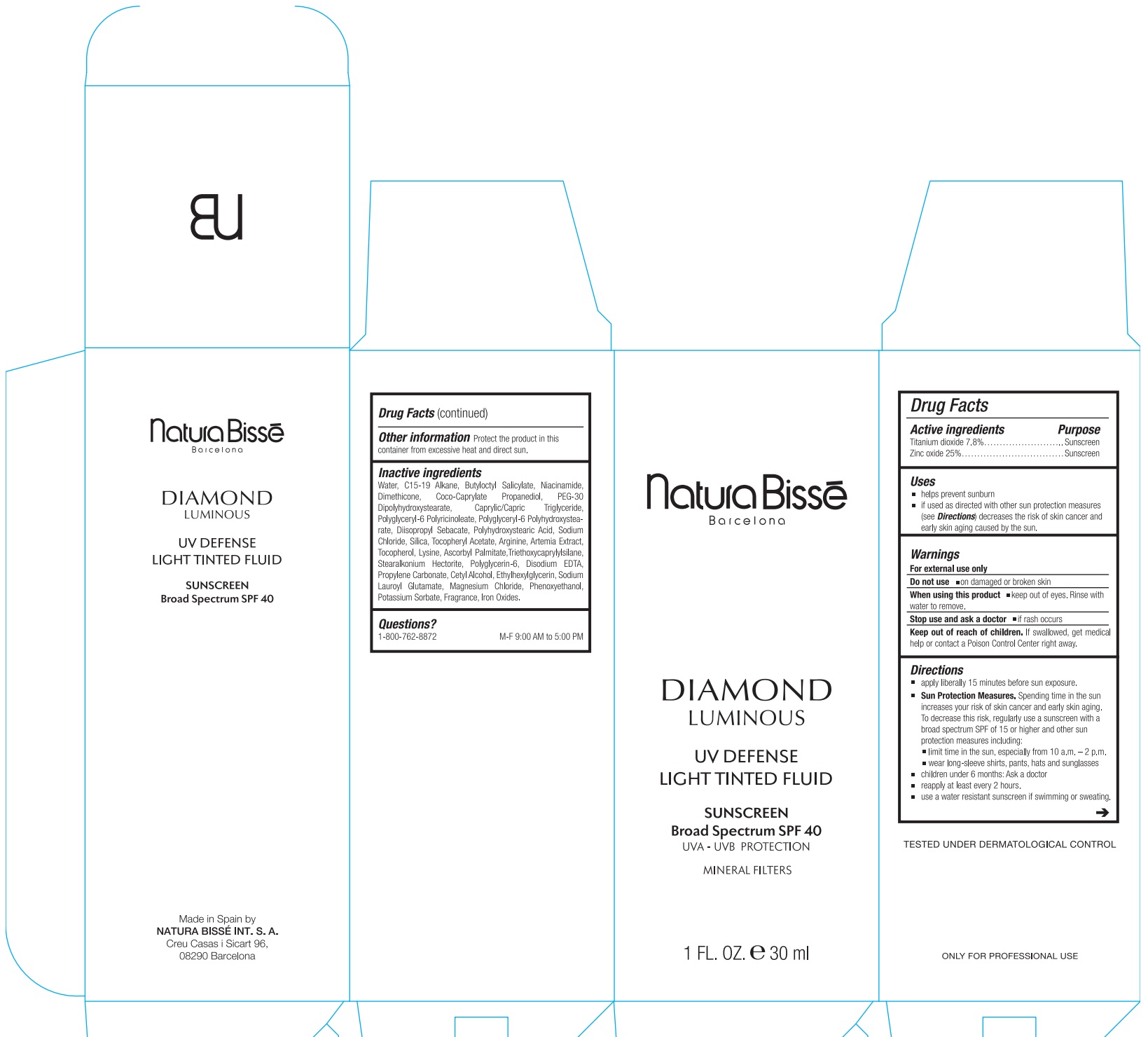
Label: DIAMOND LUMINOUS UV DEFENSE LIGHT TINTED FLUID SUNSCREEN BROAD SPECTRUM SPF 40- titanium dioxide, zinc oxide lotion
- NDC Code(s): 63730-001-01, 63730-001-02, 63730-001-03, 63730-001-04
- Packager: NATURA BISSE INTERNATIONAL SA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
apply liberally 15 minutes before sun exposure.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- children under 6 months: Ask a doctor
- reapply at least every 2 hours.
- use a water resistant sunscreen if swimming or sweating.
- Other information
-
Inactive ingredients
Water, C15-19 Alkane, Butyloctyl Salicylate, Niacinamide, Dimethicone, Coco-Caprylate Propanediol, PEG-30 Dipolyhydroxystearate, Caprylic/Capric Triglyceride, Polyglyceryl-6 Polyricinoleate, Polyglyceryl-6 Polyhydroxystearate, Diisopropyl Sebacate, Polyhydroxystearic Acid, Sodium Chloride, Silica, Tocopheryl Acetate, Arginine, Artemia Extract, Tocopherol, Lysine, Ascorbyl Palmitate,Triethoxycaprylylsilane, Stearalkonium Hectorite, Polyglycerin-6, Disodium EDTA, Propylene Carbonate, Cetyl Alcohol, Ethylhexylglycerin, Sodium Lauroyl Glutamate, Magnesium Chloride, Phenoxyethanol, Potassium Sorbate, Fragrance, Iron Oxides.
- Questions?
- Package Labeling:63730-001-01
- Package Labeling:63730-001-02
- Package Labeling:63730-001-03
- Package Labeling:63730-001-04
-
INGREDIENTS AND APPEARANCE
DIAMOND LUMINOUS UV DEFENSE LIGHT TINTED FLUID SUNSCREEN BROAD SPECTRUM SPF 40
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63730-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) C15-19 ALKANE (UNII: CI87N1IM01) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) COCO-CAPRYLATE (UNII: 4828G836N6) PROPANEDIOL (UNII: 5965N8W85T) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) SODIUM CHLORIDE (UNII: 451W47IQ8X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ARGININE (UNII: 94ZLA3W45F) TOCOPHEROL (UNII: R0ZB2556P8) LYSINE (UNII: K3Z4F929H6) ASCORBYL PALMITATE (UNII: QN83US2B0N) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERIN-6 (UNII: M51422LRAM) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CETYL ALCOHOL (UNII: 936JST6JCN) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM LAUROYL GLUTAMATE (UNII: NCX1UU2D33) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63730-001-01 4 mL in 1 PACKET; Type 0: Not a Combination Product 06/01/2021 2 NDC:63730-001-02 1 in 1 BOX 06/01/2021 2 10 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:63730-001-03 1 in 1 BOX 06/01/2021 3 30 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:63730-001-04 1 in 1 BOX 06/01/2021 4 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2021 Labeler - NATURA BISSE INTERNATIONAL SA (464431576)