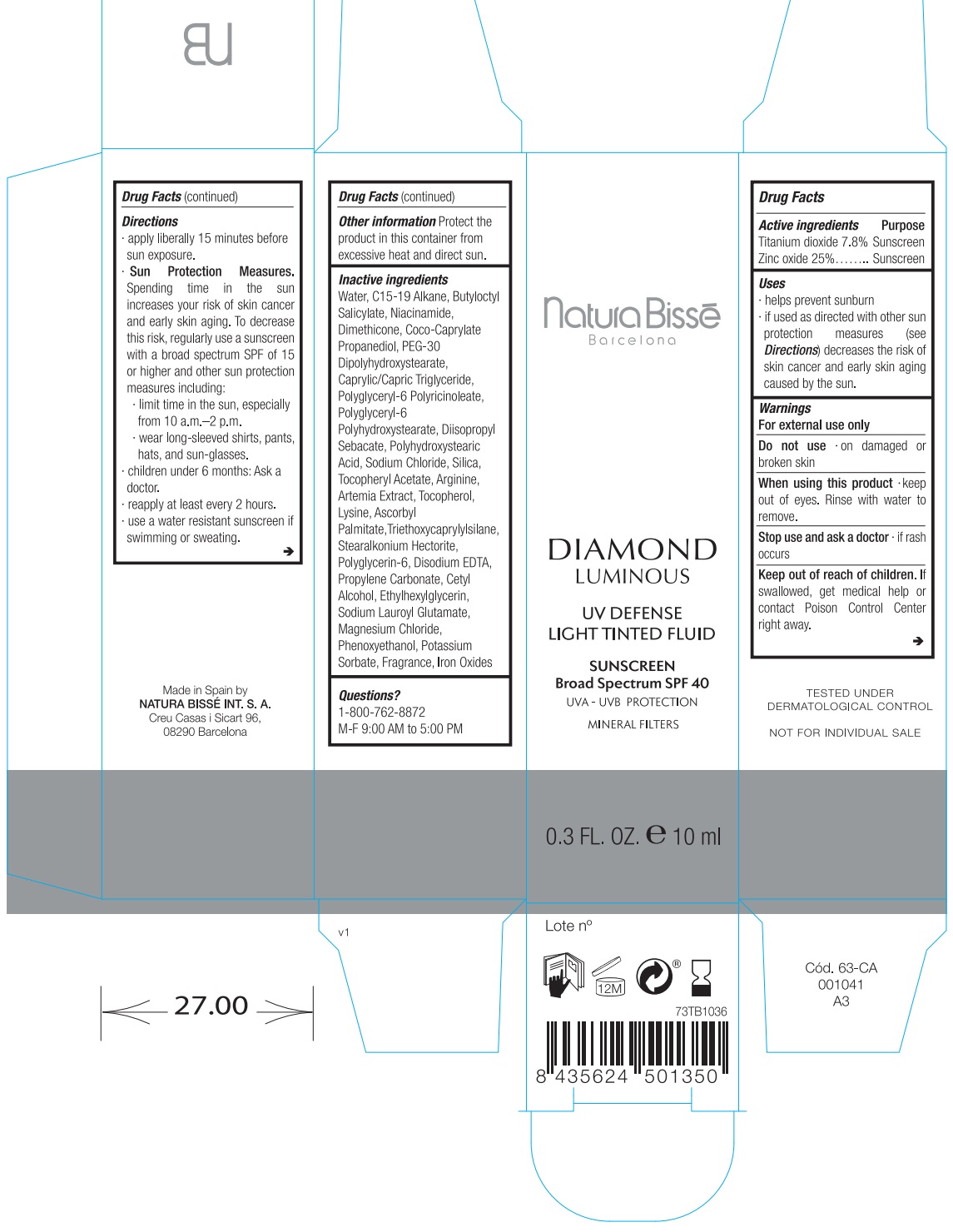
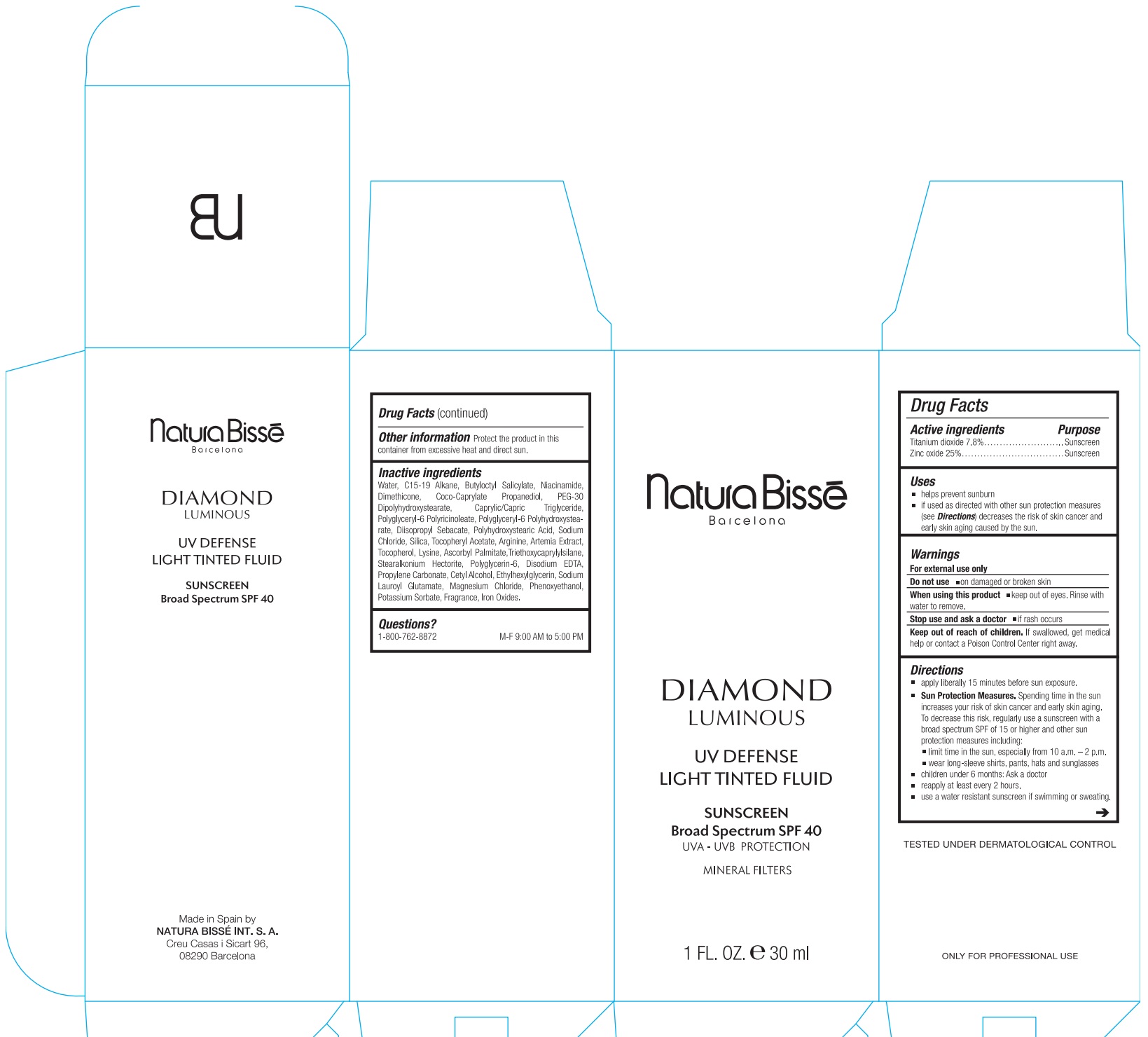
Uses
- helps prevent sunburn.
- If used as directed with other sun protection measures (see ) decreases the risk of skin cancer and early skin aging caused by the sun. Directions
Directions
apply liberally 15 minutes before sun exposure.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- children under 6 months: Ask a doctor
- reapply at least every 2 hours.
- use a water resistant sunscreen if swimming or sweating.
Inactive ingredients
Water, C15-19 Alkane, Butyloctyl Salicylate, Niacinamide, Dimethicone, Coco-Caprylate Propanediol, PEG-30 Dipolyhydroxystearate, Caprylic/Capric Triglyceride, Polyglyceryl-6 Polyricinoleate, Polyglyceryl-6 Polyhydroxystearate, Diisopropyl Sebacate, Polyhydroxystearic Acid, Sodium Chloride, Silica, Tocopheryl Acetate, Arginine, Artemia Extract, Tocopherol, Lysine, Ascorbyl Palmitate,Triethoxycaprylylsilane, Stearalkonium Hectorite, Polyglycerin-6, Disodium EDTA, Propylene Carbonate, Cetyl Alcohol, Ethylhexylglycerin, Sodium Lauroyl Glutamate, Magnesium Chloride, Phenoxyethanol, Potassium Sorbate, Fragrance, Iron Oxides.