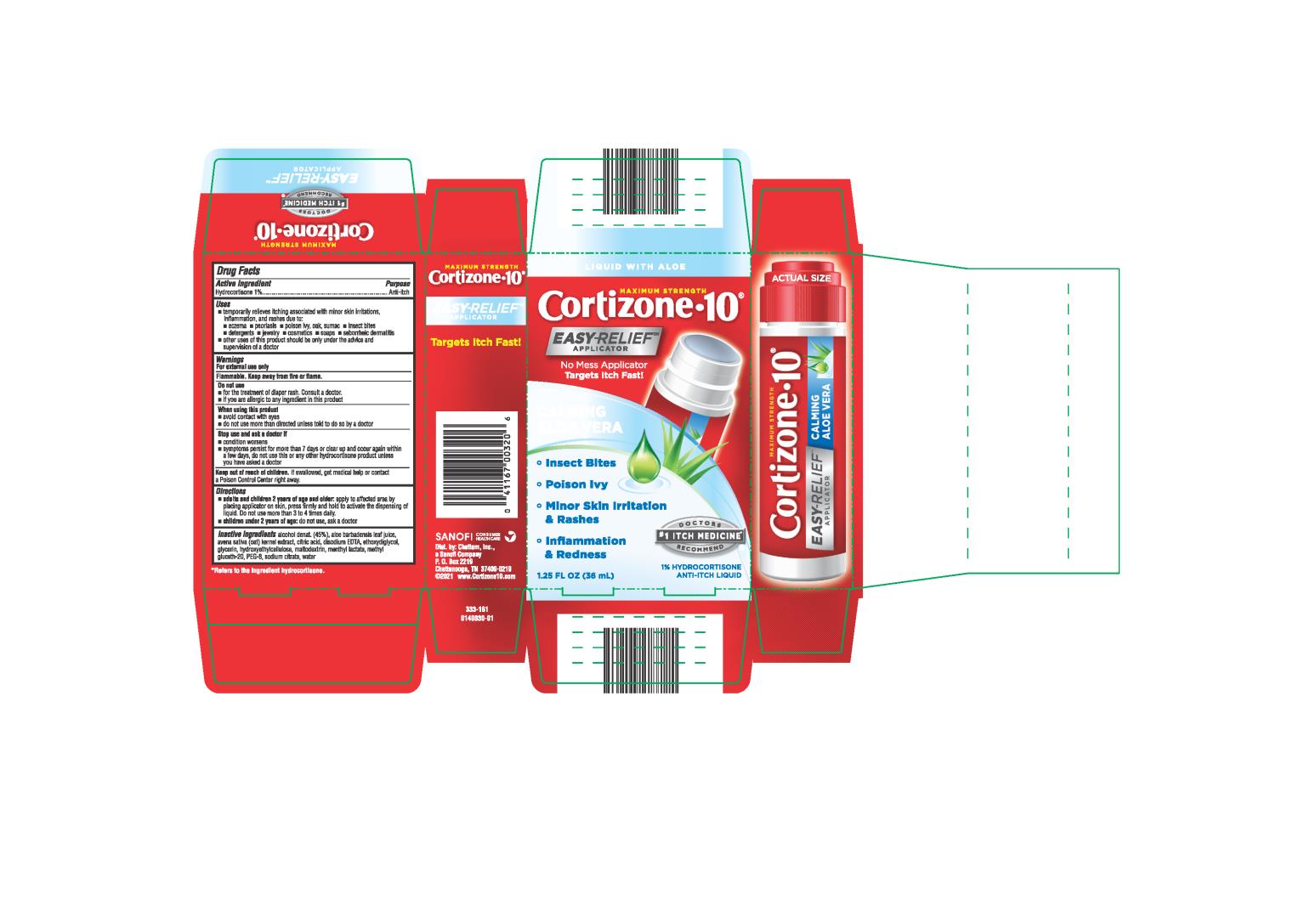
Label: CORTIZONE 10 EASY RELIEF- hydrocortisone liquid
- NDC Code(s): 41167-0032-0
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient:
- Purpose
-
Uses
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
• eczema
• psoriasis
• poison ivy, oak, sumac
• insect bites
• detergents
• jewelry
• cosmetics
• soaps
• seborrheic dermatitis
- other uses of this product should be only under the advice and supervision of a doctor
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
-
Warnings
For external use only
Flammable. Keep away from fire or flame.
Do not use
- for the treatment of diaper rash. Consult a doctor.
- if you are allergic to any ingredient in this product
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor.
- for the treatment of diaper rash. Consult a doctor.
- Directions
-
Inactive Ingredients
Alcohol denat. (45%), aloe barbadensis leaf juice, avena sativa (oat) kernel extract, citric acid, disodium EDTA, ethoxydiglycol, glycerin, hydroxyethyl cellulose, maltodextrin, menthyl lactate, methyl gluceth-20, PEG-8, , sodium citrate, water
KEEP CARTON AS IT CONTAINS IMPORTANT INFORMATION.
Close cap tightly after use. 0042734-03
*Refers to the ingredient hydrocortisone
CHATTEM®
Distributed by: Chattem, Inc.
P.O. Box 2219, Chattanooga,TN 37409-0219
U.S.A. ©2008 www.chattem.com - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CORTIZONE 10 EASY RELIEF
hydrocortisone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) OAT (UNII: Z6J799EAJK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) MALTODEXTRIN (UNII: 7CVR7L4A2D) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) ALCOHOL (UNII: 3K9958V90M) METHYL GLUCETH-20 (UNII: J3QD0LD11P) SODIUM CITRATE (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0032-0 1 in 1 CARTON 10/01/2008 1 37 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/01/2008 Labeler - Chattem, Inc. (003336013) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 003336013 analysis(41167-0032) , manufacture(41167-0032)