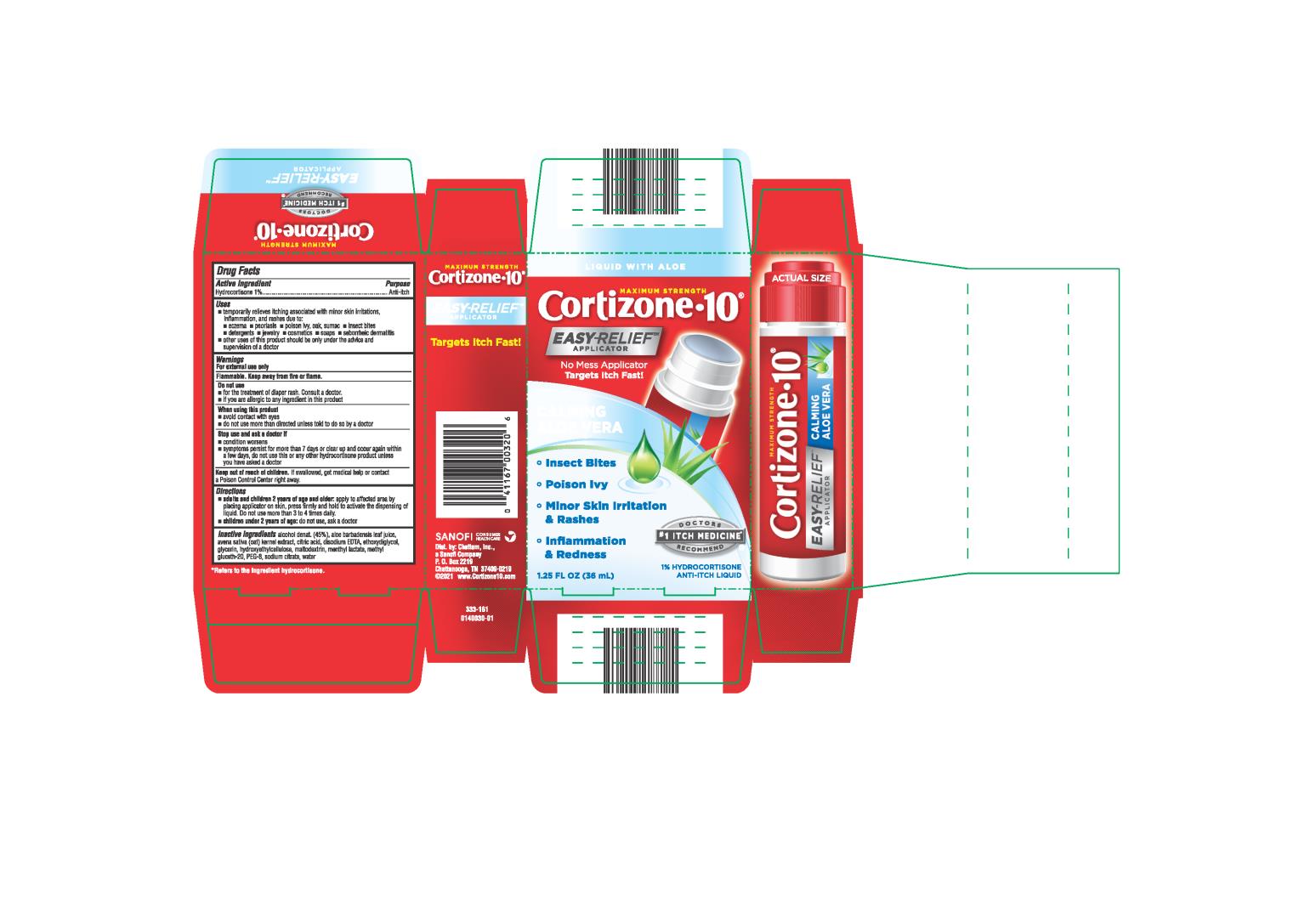
Uses
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
• eczema
• psoriasis
• poison ivy, oak, sumac
• insect bites
• detergents
• jewelry
• cosmetics
• soaps
• seborrheic dermatitis
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
Flammable. Keep away from fire or flame.
Do not use
- for the treatment of diaper rash. Consult a doctor.
- if you are allergic to any ingredient in this product
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor.
Directions
-
adults and children 2 years of age and older: apply to affected area by placing applicator on skin, press firmly and hold to activate the dispensing of liquid. Do not use more than 3 to 4 times daily.
- children under 2 years of age: do not use, ask a doctor
Inactive Ingredients
Alcohol denat. (45%), aloe barbadensis leaf juice, avena sativa (oat) kernel extract, citric acid, disodium EDTA, ethoxydiglycol, glycerin, hydroxyethyl cellulose, maltodextrin, menthyl lactate, methyl gluceth-20, PEG-8, , sodium citrate, water
KEEP CARTON AS IT CONTAINS IMPORTANT INFORMATION.
Close cap tightly after use. 0042734-03
*Refers to the ingredient hydrocortisone
CHATTEM®
Distributed by: Chattem, Inc.
P.O. Box 2219, Chattanooga,TN 37409-0219
U.S.A. ©2008 www.chattem.com