Label: PRENARA capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 69336-352-30 - Packager: Sterling-Knight Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 3, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Prenara is an orally administered prescription dietary supplement formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
Prenara is a prescription dietary supplement for use throughout pregnancy, during the prenatal and postnatal period for both lactating and non-lactating mothers and throughout the childbearing years.
Prenara should be administered under the supervision of a licensed medical practitioner.Each softgel capsule contains:
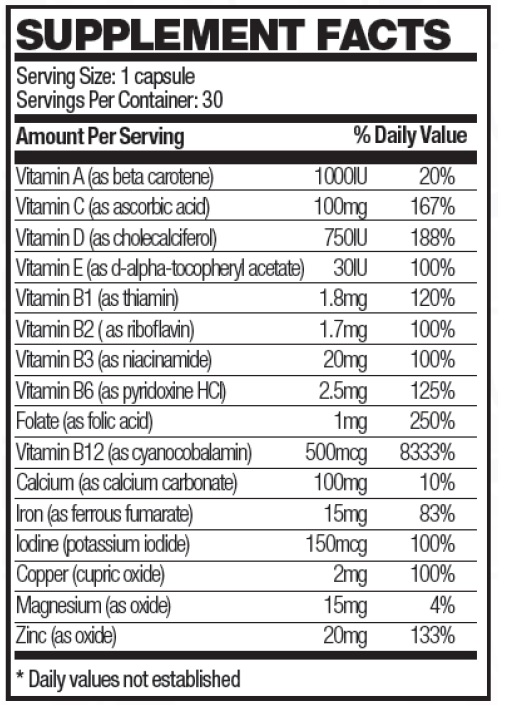
Other Ingredients: Soybean oil, gelatin (bovine), bees wax (yellow), lecithin, glycerin, deionized water, FD&C red #40, FD&C blue #1, titanium dioxide.
- INDICATIONS AND USAGE:
-
CONTRAINDICATIONS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Prenara should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications. -
WARNINGS AND PRECAUTIONS:
This product should be administered with caution in patients with a history of liver disease, jaundice or diabetes mellitus.
Folic acid above 1mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations remain progressive.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
- ADVERSE REACTION:
- DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- STORAGE:
-
SPL UNCLASSIFIED SECTION
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
Sterling-Knight Pharmaceuticals, LLC
Ripley, MS 38663
Item 35230 Rev. 0320-1 - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRENARA
prenara capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69336-352 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1000 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg VITAMIN D (UNII: 9VU1KI44GP) (CHOLECALCIFEROL - UNII:1C6V77QF41) VITAMIN D 750 [iU] THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.8 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.7 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 2.5 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 0.5 mg CALCIUM (UNII: SY7Q814VUP) (CALCIUM - UNII:SY7Q814VUP) CALCIUM 100 mg IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 0.15 mg COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 2 mg MAGNESIUM (UNII: I38ZP9992A) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM 15 mg ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 20 mg Product Characteristics Color purple Score no score Shape capsule Size 11mm Flavor Imprint Code 352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69336-352-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/09/2020 Labeler - Sterling-Knight Pharmaceuticals, LLC (079556942) Establishment Name Address ID/FEI Business Operations Sterling-Knight Pharmaceuticals, LLC 079556942 manufacture(69336-352) , label(69336-352)




