DESCRIPTION:
Prenara is an orally administered prescription dietary supplement formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
Prenara is a prescription dietary supplement for use throughout pregnancy, during the prenatal and postnatal period for both lactating and non-lactating mothers and throughout the childbearing years.
Prenara should be administered under the supervision of a licensed medical practitioner.
Each softgel capsule contains:
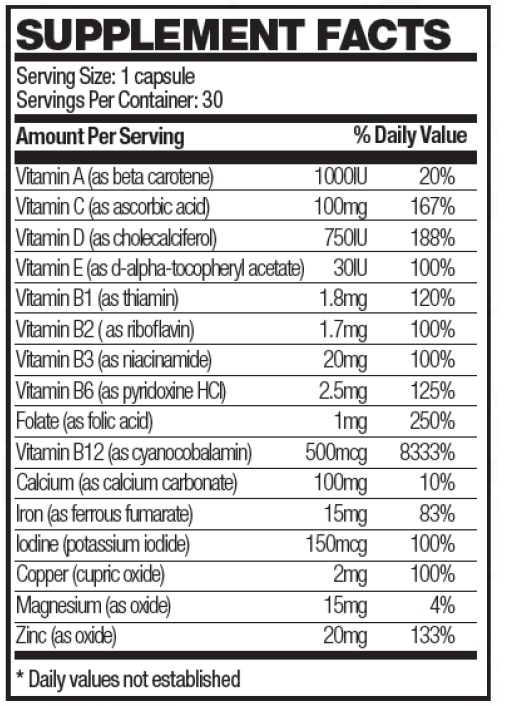
Other Ingredients: Soybean oil, gelatin (bovine), bees wax (yellow), lecithin, glycerin, deionized water, FD&C red #40, FD&C blue #1, titanium dioxide.
INDICATIONS AND USAGE:
Prenara is a prescription dietary supplement for use throughout pregnancy, during the prenatal and postnatal period for both lactating and non-lactating mothers and throughout the childbearing years
CONTRAINDICATIONS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Prenara should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
WARNINGS AND PRECAUTIONS:
This product should be administered with caution in patients with a history of liver disease, jaundice or diabetes mellitus.
Folic acid above 1mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations remain progressive.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
ADVERSE REACTION:
Allergic sensitization has been reported following both oral and parental administration of folic acid.
DOSAGE AND ADMINISTRATION:
Usual dose is 1 to 2 capsules daily or as prescribed by licensed medical practitioner.
HOW SUPPLIED:
Prenara is supplied as a purple softgel capsules with 352 printed and dispensed in white HDPE plastic bottles of 30ct.
69336-352-30
STORAGE:
Store at controlled room temperature 15°-30°C (59°F-86°F). Keep in cool dry place. Call your doctor about side effects. You may report side effects to FDA at 1-800-FDA-1088. KEEP THIS OUT OF THE REACH OF CHILDREN.
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
Sterling-Knight Pharmaceuticals, LLC
Ripley, MS 38663
Item 35230 Rev. 0320-1

