Label: DOXORUBICIN HYDROCHLORIDE injectable, liposomal
- NDC Code(s): 49315-008-03, 49315-009-07
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 1, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
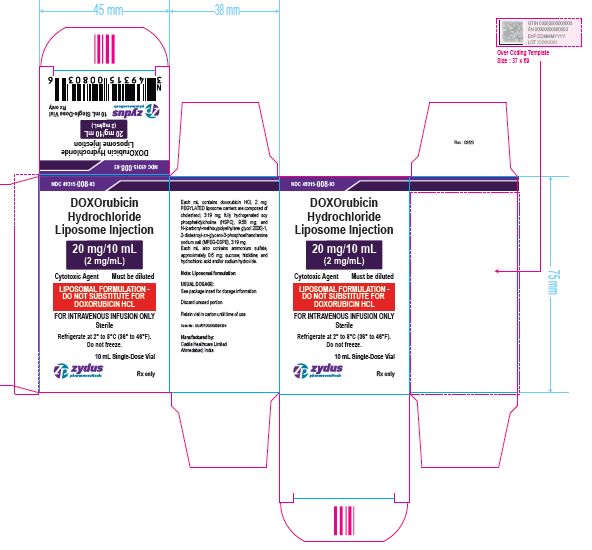
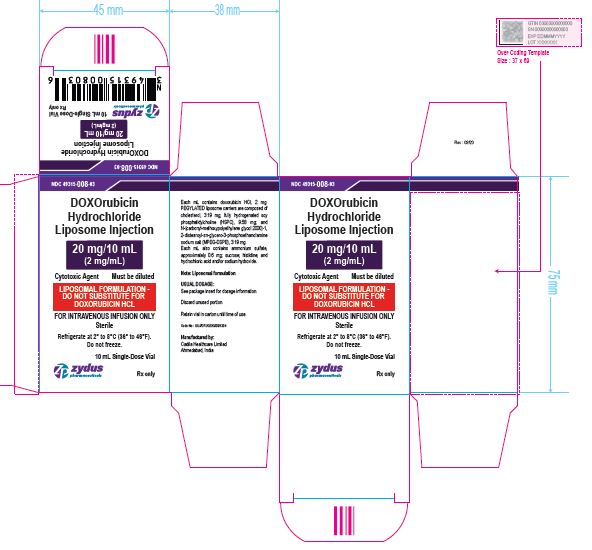
NDC 49315-008-03
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
10 mL Single-Dose Vial
Rx only

NDC 49315-008-03
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
Sterile
Refrigerate at 2⁰ to 8℃ (36⁰ to 46℉)
Do not freeze
10 mL Single-Dose Vial
Rx only
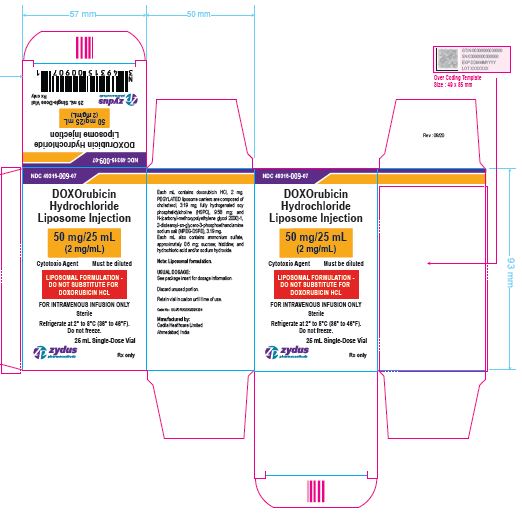
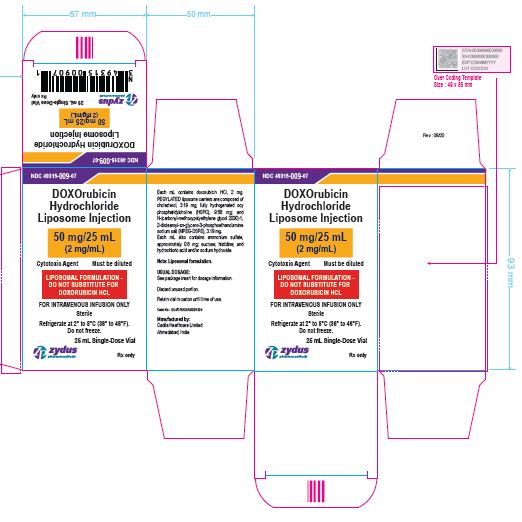
NDC 49315-009-07
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
25 mL Single-Dose Vial
Rx only

NDC 49315-009-07
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
Sterile
Refrigerate at 2⁰ to 8℃ (36⁰ to 46℉)
Do not freeze
25 mL Single-Dose Vial
Rx only
-
INGREDIENTS AND APPEARANCE
DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injectable, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49315-008 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength N-(CARBONYL-METHOXYPOLYETHYLENE GLYCOL 2000)-1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOETHANOLAMINE, SODIUM SALT (UNII: 3L6NN8ZZKU) 3.19 mg in 1 mL HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) 9.58 mg in 1 mL CHOLESTEROL (UNII: 97C5T2UQ7J) 3.19 mg in 1 mL AMMONIUM SULFATE (UNII: SU46BAM238) 0.6 mg in 1 mL SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49315-008-03 1 in 1 CARTON 09/14/2020 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212299 09/14/2020 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injectable, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49315-009 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength N-(CARBONYL-METHOXYPOLYETHYLENE GLYCOL 2000)-1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOETHANOLAMINE, SODIUM SALT (UNII: 3L6NN8ZZKU) 3.19 mg in 1 mL HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) 9.58 mg in 1 mL CHOLESTEROL (UNII: 97C5T2UQ7J) 3.19 mg in 1 mL AMMONIUM SULFATE (UNII: SU46BAM238) 0.6 mg in 1 mL SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49315-009-07 1 in 1 CARTON 09/14/2020 1 25 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212299 09/14/2020 Labeler - Zydus Lifesciences Limited (650348852) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650348852 ANALYSIS(49315-008, 49315-009) , MANUFACTURE(49315-008, 49315-009)