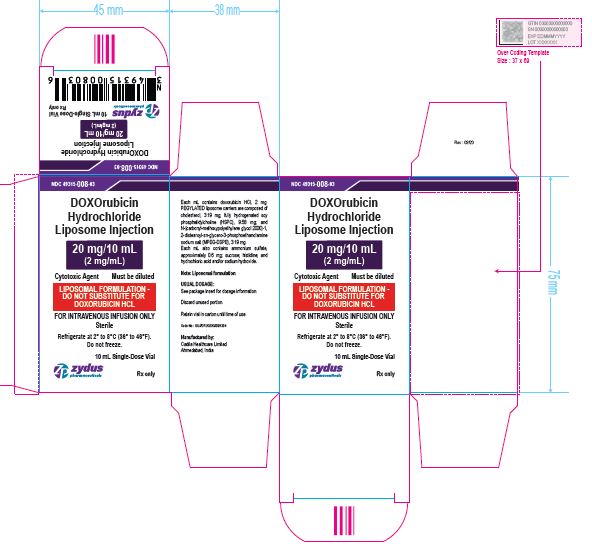
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 49315-008-03
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
10 mL Single-Dose Vial
Rx only

NDC 49315-008-03
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
Sterile
Refrigerate at 2⁰ to 8℃ (36⁰ to 46℉)
Do not freeze
10 mL Single-Dose Vial
Rx only
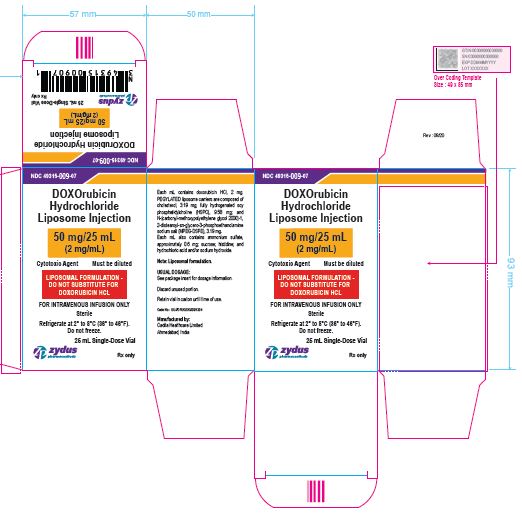
NDC 49315-009-07
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
25 mL Single-Dose Vial
Rx only

NDC 49315-009-07
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
Sterile
Refrigerate at 2⁰ to 8℃ (36⁰ to 46℉)
Do not freeze
25 mL Single-Dose Vial
Rx only