Label: GAS RELIEF ULTRA STRENGTH- simethicone capsule, liquid filled
- NDC Code(s): 69842-832-20, 69842-832-60
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each softgel)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
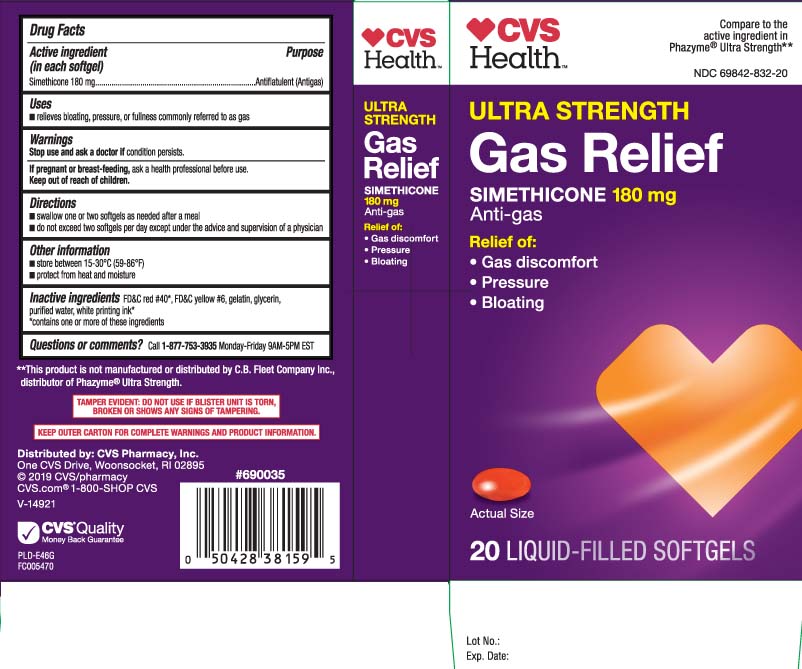
Principal Display Panel
Compare to the active ingredient in Phazyme® Ultra Strength**
ULTRA STRENGTH
GAS RELIEF
SIMETHICONE 180 mg
Anti-gas
Relief of:
- Gas discomfort
- Pressure
- Bloating
SOFTGEL
LIQUID-FILLED SOFTGELS
**This product is not manufactured or distributed by C.B. Fleet Company Inc., distributor of Phazyme® Ultra Strength.
TAMPER EVIDENT: DO NOT USE IF BLISTER UNIT IS TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
- Package Label
-
INGREDIENTS AND APPEARANCE
GAS RELIEF ULTRA STRENGTH
simethicone capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-832 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 180 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color orange Score no score Shape OVAL Size 12mm Flavor Imprint Code S180;05A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-832-60 1 in 1 BOX 12/31/2018 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69842-832-20 1 in 1 BOX 12/31/2018 2 20 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 12/31/2018 Labeler - CVS Pharmacy (062312574)