Directions
- swallow one or two softgels as needed after a meal
- do not exceed two softgels per day except under the advice and supervision of a physician
Inactive ingredients
FD&C red #40*, FD&C yellow #6, gelatin, glycerin, purified water, white printing ink*
*contains one or more of these ingredients
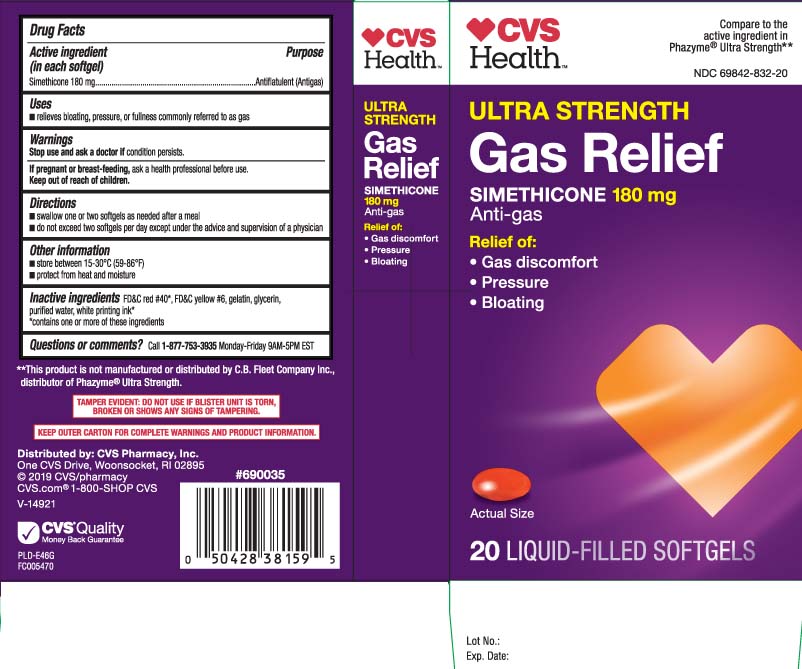
Principal Display Panel
Compare to the active ingredient in Phazyme® Ultra Strength**
ULTRA STRENGTH
GAS RELIEF
SIMETHICONE 180 mg
Anti-gas
Relief of:
- Gas discomfort
- Pressure
- Bloating
SOFTGEL
LIQUID-FILLED SOFTGELS
**This product is not manufactured or distributed by C.B. Fleet Company Inc., distributor of Phazyme® Ultra Strength.
TAMPER EVIDENT: DO NOT USE IF BLISTER UNIT IS TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895