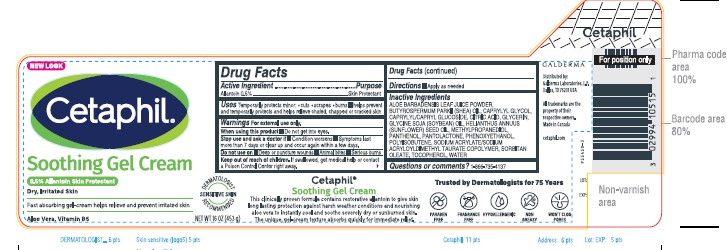
Label: CETAPHIL SOOTHING GEL CREAM- allantoin cream
- NDC Code(s): 0299-4105-00, 0299-4105-05, 0299-4105-10, 0299-4105-15
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients......Purpose
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Warnings
For external use only.
When using this product ■ Do not get into eyes.
Stop use and ask a Doctor if ● condition worsens ● symptoms last more than 7 days or clear up and occur again within a few days.
Do not use on ■Deep or puncture wounds
■Animal bites ■Serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Directions
-
Inactive Ingredients
ALOE BARBADENSIS LEAF JUICE POWDER, BUTYROSPERMUM PARKII (SHEA) OIL, CAPRYLYL GLYCOL, CAPRYLYL/CAPRYL GLUCOSIDE, CITRIC ACID, GLYCERIN, GLYCINE SOJA (SOYBEAN) OIL, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, METHYLPROPANEDIOL, PANTHENOL, PANTOLACTONE, PHENOXYETHANOL, POLYISOBUTENE, SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, SORBITAN OLEATE, TOCOPHEROL, WATER
- Questions or comments?
-
PRINCIPLE DISPLAY PANEL - 16 OZ jar
NEW LOOK
Cetaphil®
Soothing Gel Cream
0.5% Allantoin Skin ProtectantDry, Irritated Skin
Fast absorbing gel-cream
helps relieves and prevent irritated skin.
Aloe Vera, Vitamin B5
Dermatologist Recommended
Sensitive Skin
NET WT 16 OZ (453 g)
Distributed By:
Galderma Laboratories, L.P.
Dallas, TX 75201 USAAll trademarks are the property of their respective owners.
Made in Canada.
cetaphil.com
P55450-3
-
INGREDIENTS AND APPEARANCE
CETAPHIL SOOTHING GEL CREAM
allantoin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Allantoin (UNII: 344S277G0Z) (Allantoin - UNII:344S277G0Z) Allantoin 5 mg in 1 g Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Sheanut Oil (UNII: O88E196QRF) Caprylyl Glycol (UNII: 00YIU5438U) Caprylyl/Capryl Oligoglucoside (UNII: E00JL9G9K0) Citric Acid Monohydrate (UNII: 2968PHW8QP) Glycerin (UNII: PDC6A3C0OX) Soybean Oil (UNII: 241ATL177A) Sunflower Oil (UNII: 3W1JG795YI) Methylpropanediol (UNII: N8F53B3R4R) Panthenol (UNII: WV9CM0O67Z) Pantolactone, (R)- (UNII: J288D7O0JS) Phenoxyethanol (UNII: HIE492ZZ3T) Polyisobutylene (1000 Mw) (UNII: 5XB3A63Y52) Sodium Acrylate/Sodium Acryloyldimethyltaurate Copolymer (4000000 Mw) (UNII: 1DXE3F3OZX) Tocopherol (UNII: R0ZB2556P8) Water (UNII: 059QF0KO0R) Sorbitan Monooleate (UNII: 06XEA2VD56) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4105-00 10 g in 1 TUBE; Type 0: Not a Combination Product 02/17/2020 2 NDC:0299-4105-05 85 g in 1 TUBE; Type 0: Not a Combination Product 02/17/2020 3 NDC:0299-4105-10 226 g in 1 TUBE; Type 0: Not a Combination Product 02/17/2020 4 NDC:0299-4105-15 453 g in 1 JAR; Type 0: Not a Combination Product 02/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/17/2020 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(0299-4105)