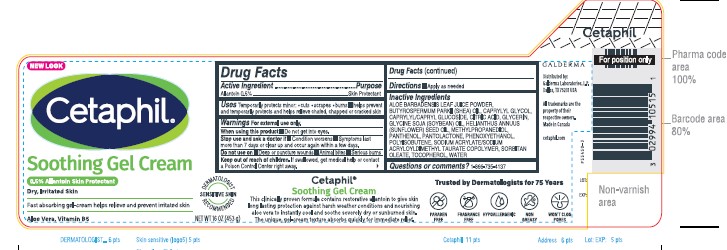
Uses
Temporarily protects minor: ●cuts
●scrapes ●burns ■helps prevent and temporarily
protects and helps relieve chafed, chapped or
cracked skin
Warnings
For external use only.
When using this product ■ Do not get into eyes.
Stop use and ask a Doctor if ● condition worsens ● symptoms last more than 7 days or clear up and occur again within a few days.
Do not use on ■Deep or puncture wounds
■Animal bites ■Serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
ALOE BARBADENSIS LEAF JUICE POWDER, BUTYROSPERMUM PARKII (SHEA) OIL, CAPRYLYL GLYCOL, CAPRYLYL/CAPRYL GLUCOSIDE, CITRIC ACID, GLYCERIN, GLYCINE SOJA (SOYBEAN) OIL, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, METHYLPROPANEDIOL, PANTHENOL, PANTOLACTONE, PHENOXYETHANOL, POLYISOBUTENE, SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, SORBITAN OLEATE, TOCOPHEROL, WATER
PRINCIPLE DISPLAY PANEL - 16 OZ jar
NEW LOOK
Cetaphil®
Soothing Gel Cream
0.5% Allantoin Skin Protectant
Dry, Irritated Skin
Fast absorbing gel-cream
helps relieves and prevent irritated skin.
Aloe Vera, Vitamin B5
Dermatologist Recommended
Sensitive Skin
NET WT 16 OZ (453 g)
Distributed By:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
Made in Canada.
cetaphil.com
P55450-3