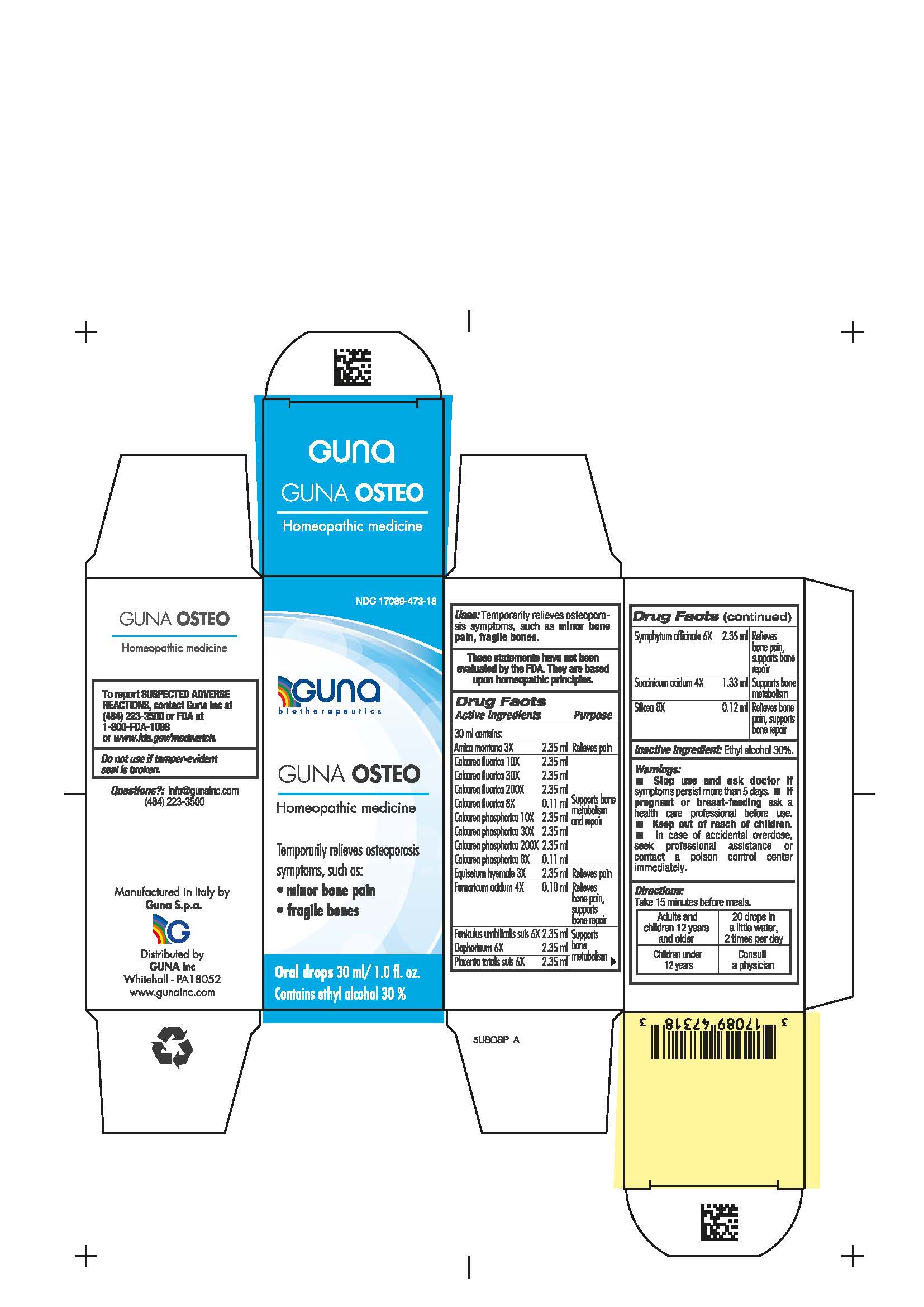
Label: GUNA OSTEO- arnica montana root - calcium fluoride - calcium phosphate - equisetum hyemale - fumaric acid - sus scrofa umbilical cord - sus scrofa ovary - sus scrofa placenta - comfrey root - succinic acid - silicon dioxide - solution/ drops
- NDC Code(s): 17089-473-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 17, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- DIRECTIONS
- WARNINGS
- PREGNANCY
-
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days.
If pregnant or breast-feeding ask a health care professional before use.
Keep out of reach of children. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Contains ethyl alcohol 30% - USES
-
ACTIVE INGREDIENTS/PURPOSE
Arnica montana 3X Relieves pain
Calcarea fluorica 8X, 10X, 30X, 200X Supports bone metabolism and repair
Calcarea phosphorica 8X, 10X, 30X, 200X Supports bone metabolism and repair
Equisetum hyemale 3X Relieves pain
Fumaricum acidum 4X Relieves bone pain, supports bone repair
Funiculus umbilicalis suis 6X Supports bone metabolism
Oophorinum 6X Supports bone metabolism
Placenta totalis suis 6X Supports bone metabolism
Symphytum officinale 6X Relieves bone pain, supports bone repair
Succinicum acidum 4X Supports bone metabolism
Silicea 8X Relieves bone pain, supports bone repair
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA OSTEO
arnica montana root - calcium fluoride - calcium phosphate - equisetum hyemale - fumaric acid - sus scrofa umbilical cord - sus scrofa ovary - sus scrofa placenta - comfrey root - succinic acid - silicon dioxide - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-473 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 3 [hp_X] in 30 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 4 [hp_X] in 30 mL SUS SCROFA UMBILICAL CORD (UNII: 118OYG6W3H) (SUS SCROFA UMBILICAL CORD - UNII:118OYG6W3H) SUS SCROFA UMBILICAL CORD 6 [hp_X] in 30 mL SUS SCROFA OVARY (UNII: S7YTV04R8O) (SUS SCROFA OVARY - UNII:S7YTV04R8O) SUS SCROFA OVARY 6 [hp_X] in 30 mL SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 6 [hp_X] in 30 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 30 mL SUCCINIC ACID (UNII: AB6MNQ6J6L) (SUCCINIC ACID - UNII:AB6MNQ6J6L) SUCCINIC ACID 4 [hp_X] in 30 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 8 [hp_X] in 30 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 8 [hp_X] in 30 mL CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM PHOSPHATE 8 [hp_X] in 30 mL EQUISETUM HYEMALE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE 3 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-473-18 1 in 1 BOX 02/17/2021 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/17/2021 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-473)