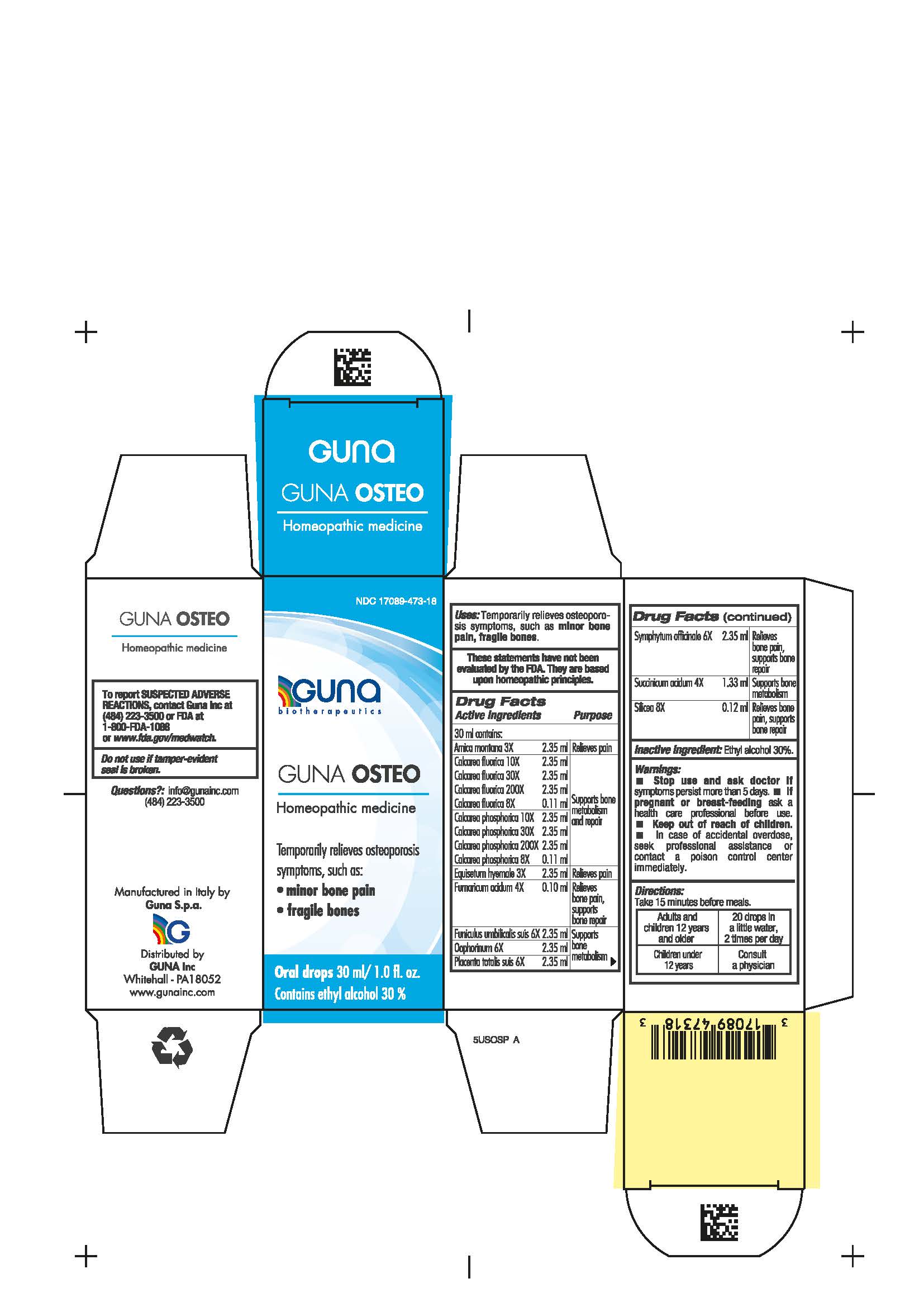
DIRECTIONS
Adults and children 12 years and older 20 drops in a little water, 2 times per day
Children under 12 years of age consult a physician
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days.
If pregnant or breast-feeding ask a health care professional before use.
Keep out of reach of children. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Contains ethyl alcohol 30%
ACTIVE INGREDIENTS/PURPOSE
Arnica montana 3X Relieves pain
Calcarea fluorica 8X, 10X, 30X, 200X Supports bone metabolism and repair
Calcarea phosphorica 8X, 10X, 30X, 200X Supports bone metabolism and repair
Equisetum hyemale 3X Relieves pain
Fumaricum acidum 4X Relieves bone pain, supports bone repair
Funiculus umbilicalis suis 6X Supports bone metabolism
Oophorinum 6X Supports bone metabolism
Placenta totalis suis 6X Supports bone metabolism
Symphytum officinale 6X Relieves bone pain, supports bone repair
Succinicum acidum 4X Supports bone metabolism
Silicea 8X Relieves bone pain, supports bone repair