Label: LESS DROWSY MOTION SICKNESS RELIEF- meclizine hydrochloride tablet
- NDC Code(s): 53943-826-29
- Packager: Discount Drug Mart
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
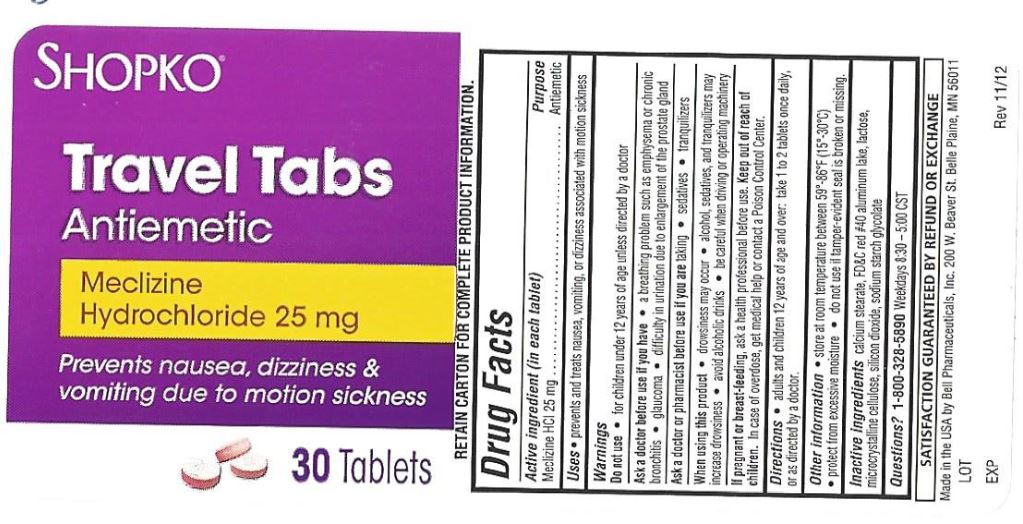
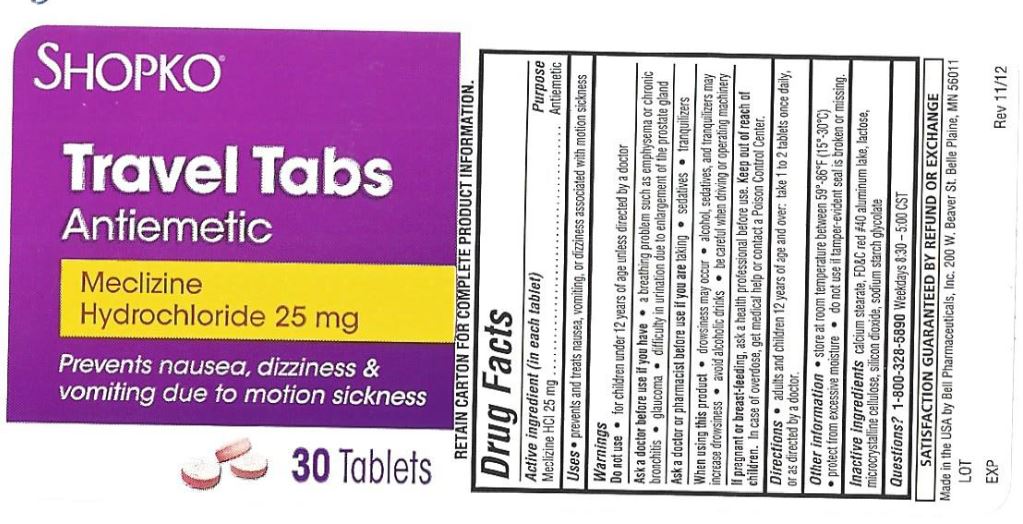
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urinating due to an enlargement of the prostate gland
- Directions
- Inactive ingredients
- Other Information
-
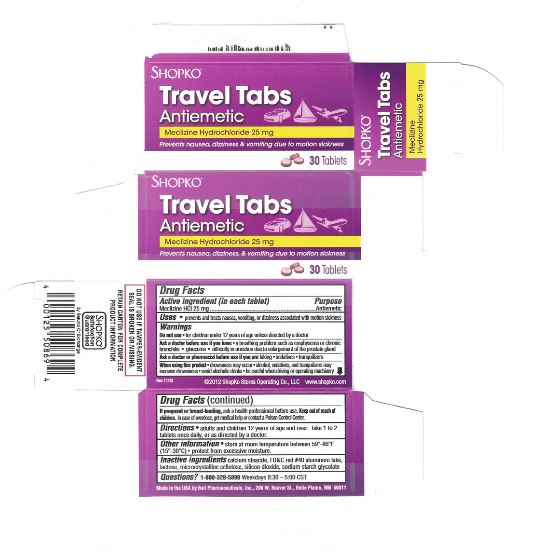
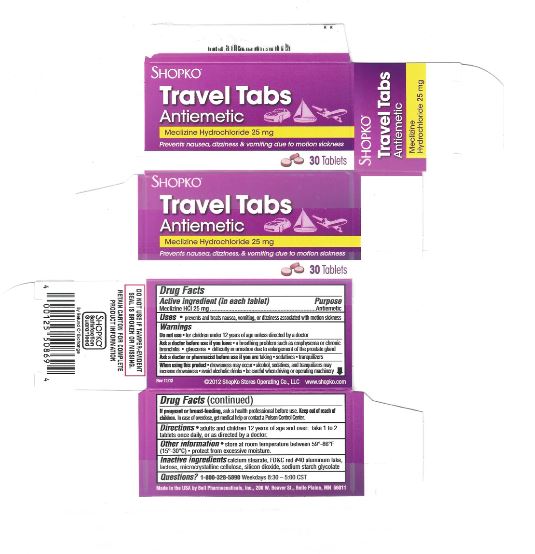
PRINCIPAL DISPLAY PANEL
Compare to Dramamine® LESS DROWSY FORMULA active ingredients*
DISCOUNT drug mart FOOD FAIR
less drowsy
motion sickness relief tabs
Meclizine Hydrochloride
- antiemetic
provides Up To 24 Hours of Motion Sickness Protection
prevents nausea, dizziness, vomiting
8 TABLETS (25 mg EACH) new smaller sizeRetain carton for complete product information.
Distributed by: Drug Mart-Food Fair
211 Commerce Dr., Medina, Ohio 44256
Questions?: 866-467-2748
-
INGREDIENTS AND APPEARANCE
LESS DROWSY MOTION SICKNESS RELIEF
meclizine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-826 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) CALCIUM STEARATE (UNII: 776XM7047L) LACTOSE (UNII: J2B2A4N98G) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color pink Score no score Shape ROUND Size 7mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-826-29 8 in 1 BLISTER PACK 12/01/2014 1 1 in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 12/01/2014 Labeler - Discount Drug Mart (047741335) Establishment Name Address ID/FEI Business Operations Bell Pharmaceuticals, Inc. 140653770 manufacture(53943-826)