Label: MAXIMUM STRENGTH CORTISONE CREAM WITH ALOE- hydrocortisone cream cream
- NDC Code(s): 69842-630-01, 69842-630-02, 69842-630-03, 69842-630-04
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
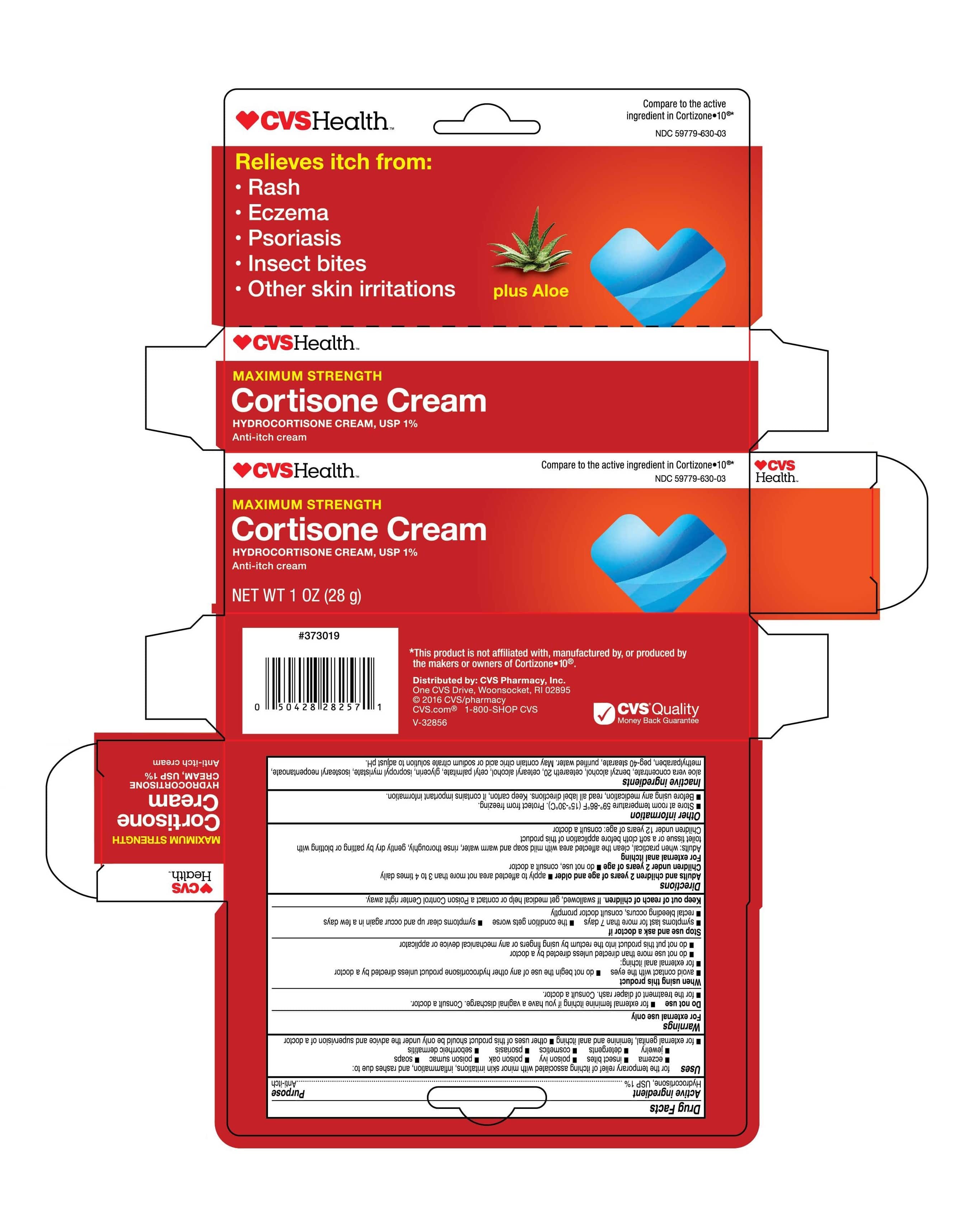
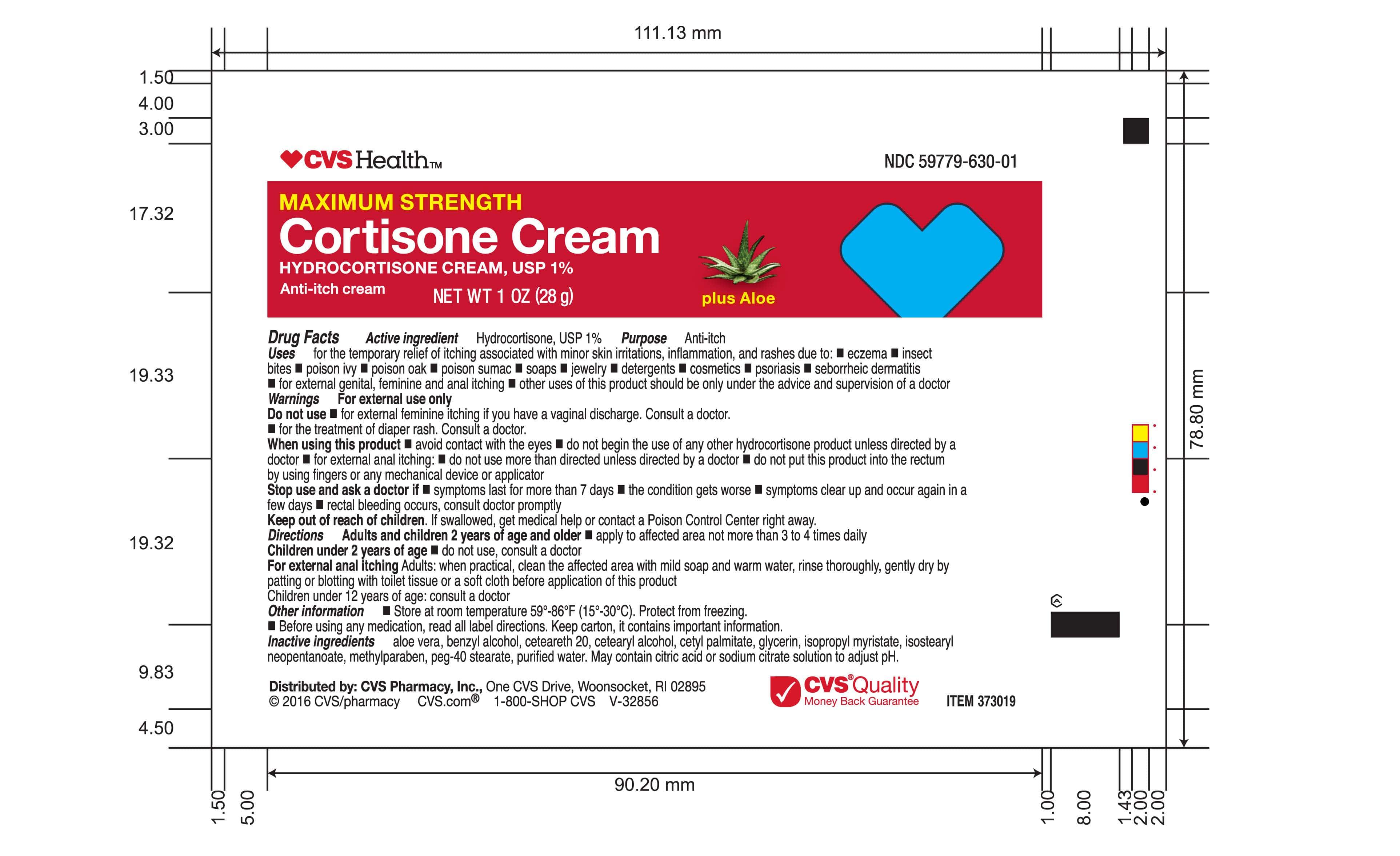
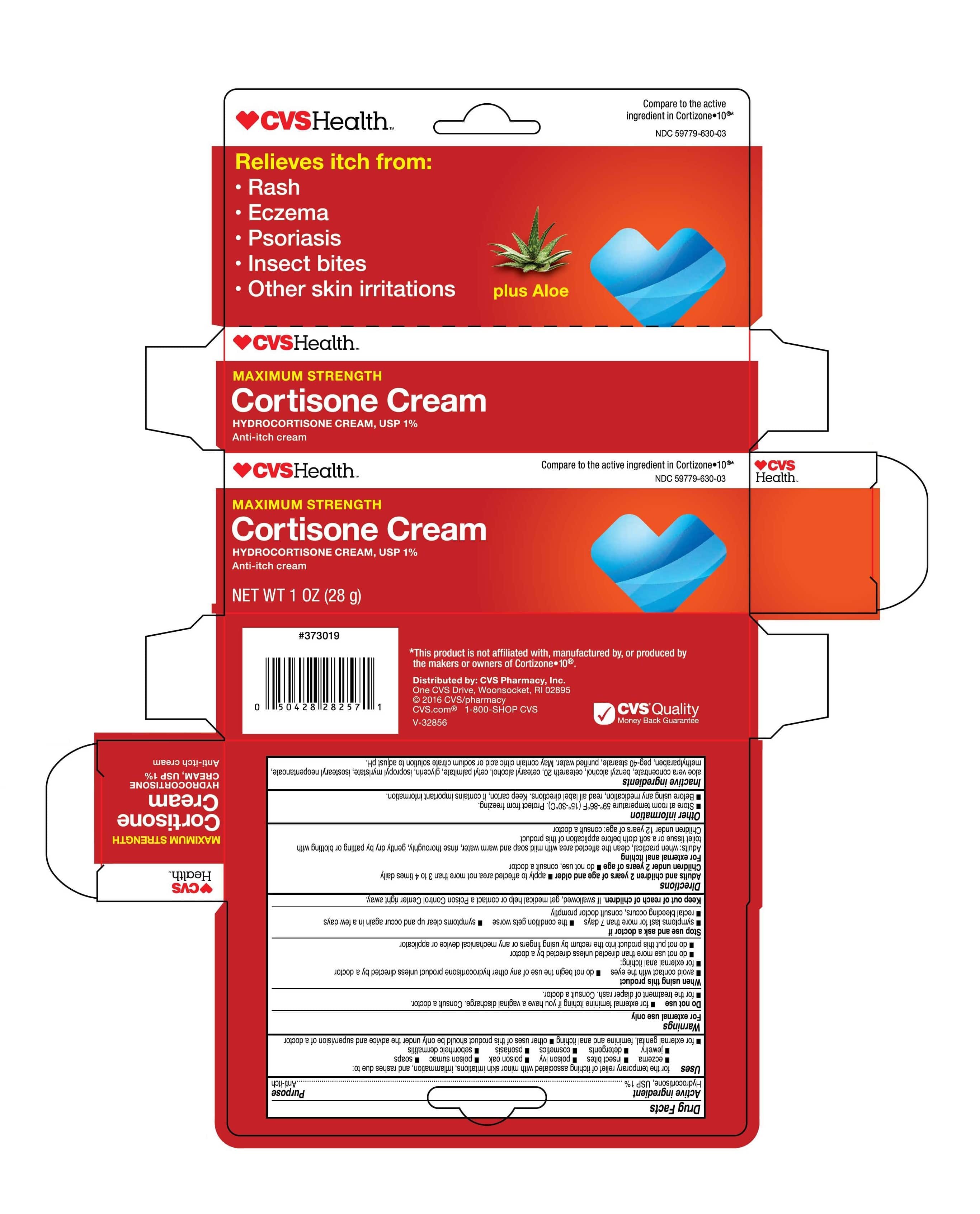
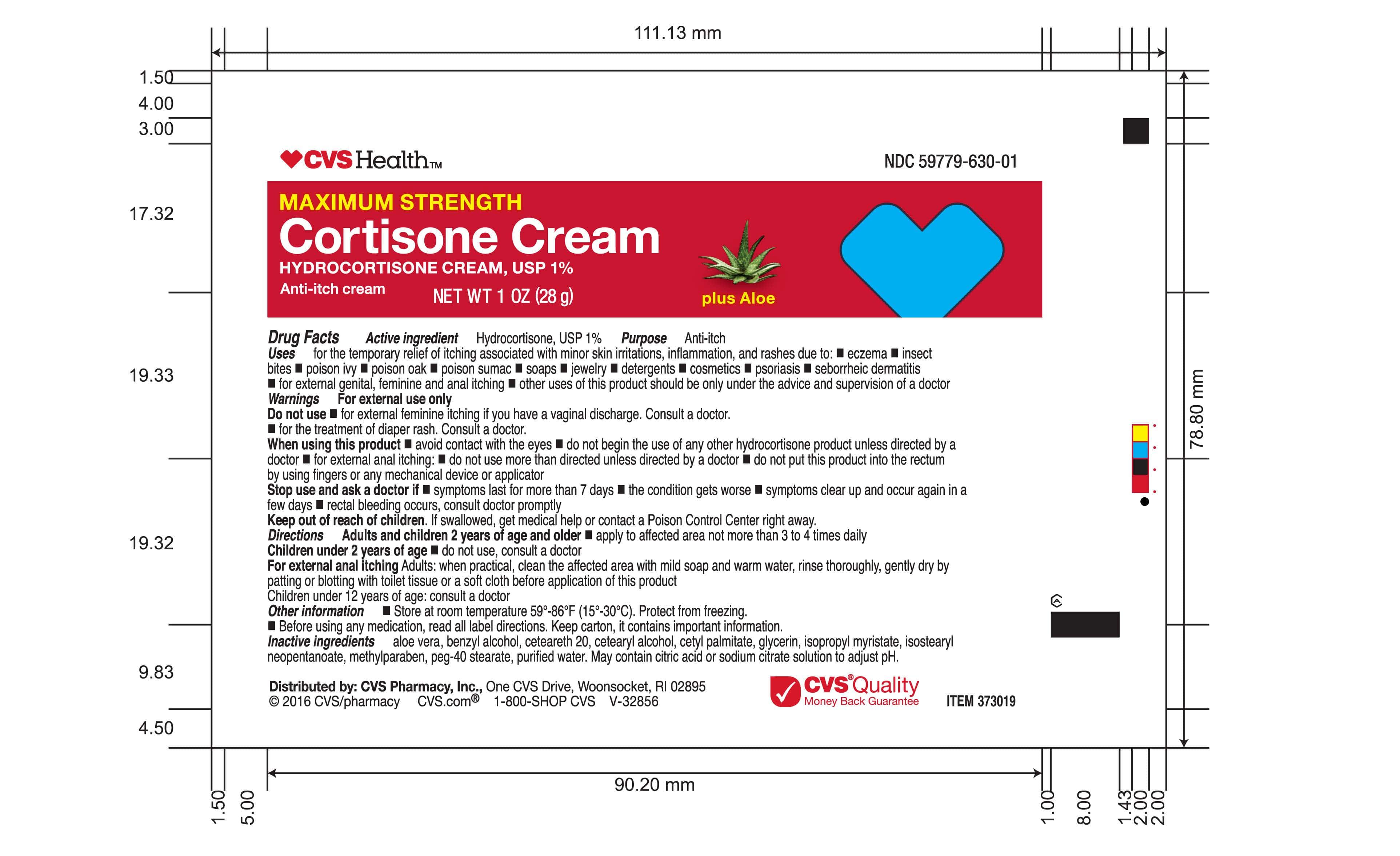
Uses
for the temporary relief of itching associated with minor skin irritations, inflammation, and rashes due to: eczema, insect bites, poison ivy, poison oak, poison sumac, soaps, jewelry, detergents, cosmetics, psoriasis, seborrheic dermatitis, for external genital, feminine and anal itching, other uses of this product should be only under the advice and supervision of a doctor
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
Adults and children 2 years of age and older * apply to affected area not more than 3 to 4 times daily.
Children under 2 years of age * do not use, consult a doctor.
For external anal itching - Adults: when practical, clean the affected area with mild soap and warm water, rinse thoroughly, gently dry by patting or blotting with toilet tissue or a soft cloth before applicaion of this product.
Children under 12 years of age: consult a doctor.
- Other information
- Inactive Ingredients
- Carton Label | 1oz
- Tube Label | 1oz
- Carton Label | 2oz
- Tube Label | 2oz
-
INGREDIENTS AND APPEARANCE
MAXIMUM STRENGTH CORTISONE CREAM WITH ALOE
hydrocortisone cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-630 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) METHYLPARABEN (UNII: A2I8C7HI9T) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PEG-40 STEARATE (UNII: ECU18C66Q7) BENZYL ALCOHOL (UNII: LKG8494WBH) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-630-03 1 in 1 CARTON 11/01/2016 1 NDC:69842-630-01 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69842-630-04 1 in 1 CARTON 11/01/2016 2 NDC:69842-630-02 56 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2016 Labeler - CVS Pharmacy (062312574) Registrant - Unipack LLC (116015769) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(69842-630)