Label: JOINTFLEX cream
- NDC Code(s): 72927-904-01, 72927-904-03, 72927-904-04
- Packager: Strides Consumer LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
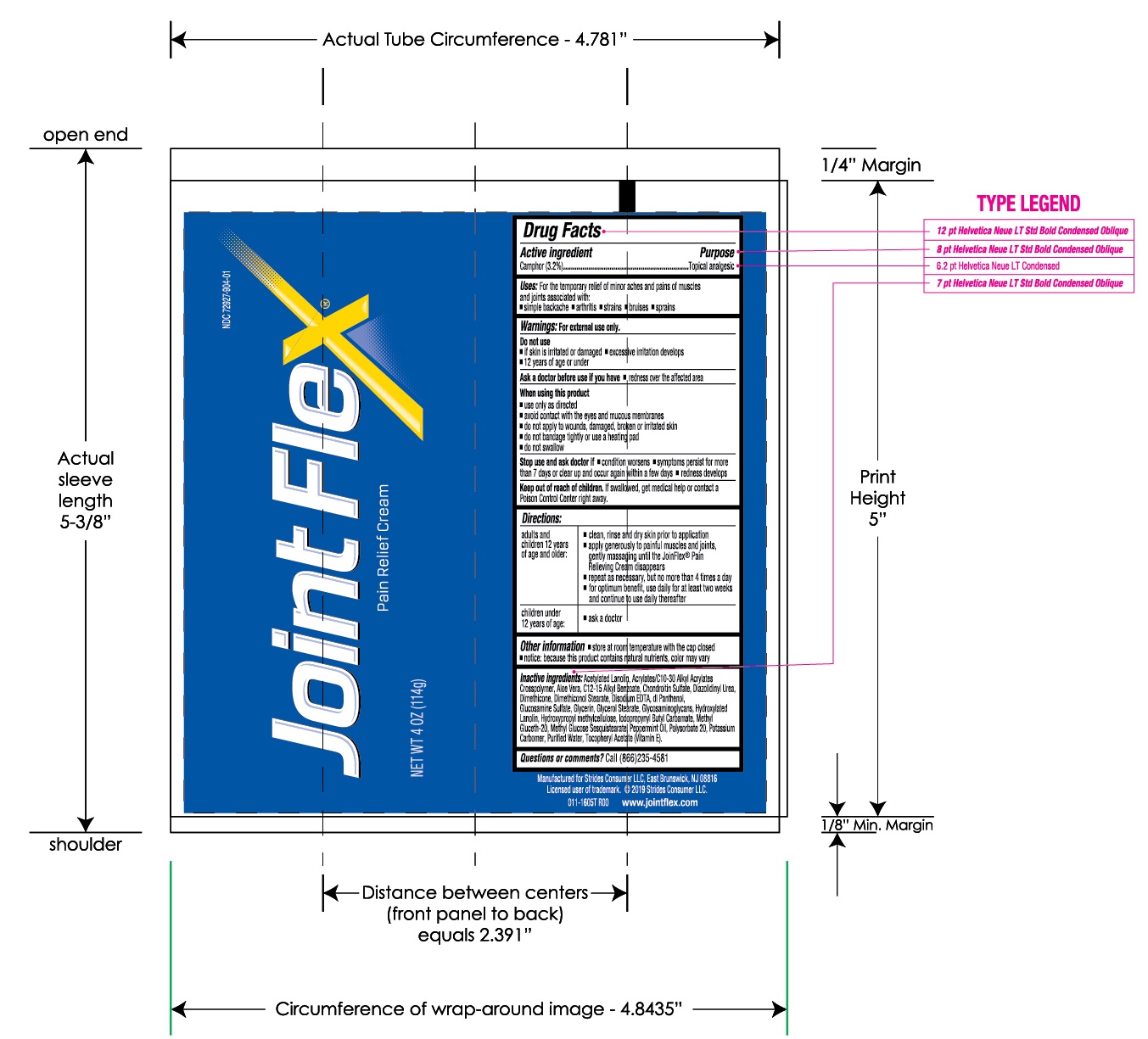
- Actice Ingredient
- Purpose
- Uses:
- Warnings:
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask doctor if
- Keep out of reach of children.
-
Directions:
adults and children 12 years of age and older:
- clean, rinse and dry skin prior to application
- apply generously to painful muscles and joints, gently massaging until the JointFlex® Pain Relieving Cream disappears
- repeat as necessary, but no more than 4 times a day
- for optimum benefit, use daily for at least two weeks and continue to use daily thereafter
children under 12 years of age:
- ask a doctor
- Other information
-
Inactive ingredients:
Acetylated Lanolin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Vera, C12-15 Alkyl Benzoate, Chondroitin Sulfate, Diazolidinyl Urea, Dimethicone, Dimethiconol Stearate, Disodium EDTA, dl Panthenol, Glucosamine Sulfate, Glycerin, Glycerol Stearate, Glycosaminoglycans, Hydroxylated Lanolin, Hydroxypropyl methycellulose, Iodopropynyl Butylcarbamate, Methyl Gluceth-20, Methyl Glucose Sesquistearate, Peppermint Oil, Polysorbate 20, Potassium Carbomer, Purified Water, Tocopheryl Acetate (Vitamin E).
-
Questions and comments?
Call (866) 235-4581 or or email stridesconsumer@emersongroup.com
Manufactured for
Strides Consumer LLC,
East Brunswick, NJ 08816
†Results based on a 2021 Healthcare Provider survey conducted by InStep Health as part of a point-of-care marketing campaign sponsored by Strides Consumer LLC.
Manufactured for Strides Consumer LLC, East Brunswick, NJ 08816
Licensed user of trademarks. © 2022 Strides Consumer LLC
www.jointflex.com
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
8 Week Challenge for pain relief
Effectively Reduces Joint Pain
#1 CLINICALLY RECOMMENDED† JOINT PAIN CREAM
JointFlex®- Pain Relief Cream
Immediate & Long Lasting Relief*
Contains Glucosamine & Chondroitin**
No stain/ No Grease/ Pleasant Scent
DEEP PENETRATING Fusome® Technology
Quick & effective penetration
Targeted pain relief
Long lasting relief when used daily
Flex Your Freedom™
Relieves Back, Neck, Shoulder, Knees, Elbow, Foot, Ankle, Leg Hand and Wrist pain
NET WT 4 OZ (114g)
NDC 72927-904-01
Visit www.jointflex.com for more information
* when applied as directed
** For skin conditioning
011-1605C-R03

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
8 Week Challenge for pain relief
Effectively Reduces Joint Pain
JointFlex®- Pain Relief Cream
Immediate & Long Lasting Relief*
Contains Glucosamine & Chondroitin**
No stain/ No Grease/ Pleasant Scent
DEEP PENETRATING Fusome® Technology
Quick & effective penetration
Targeted pain relief
Long lasting relief when used daily
Flex Your Freedom™
Relieves Back, Neck, Shoulder, Knees, Elbow, Foot, Ankle, Leg Hand and Wrist pain
NET WT 1 OZ (28g)
NDC 72927-904-03
Visit www.jointflex.com for more information
* when applied as directed
** For skin conditioning
011-1606C-R01


8 Week Challenge for pain relief
Effectively Reduces Joint Pain
JointFlex®- Pain Relief Cream
Immediate & Long Lasting Relief*
Contains Glucosamine & Chondroitin**
No stain/ No Grease/ Pleasant Scent
DEEP PENETRATING Fusome® Technology
Quick & effective penetration
Targeted pain relief
Long lasting relief when used daily
Flex Your Freedom™
Relieves Back, Neck, Shoulder, Knees, Elbow, Foot, Ankle, Leg Hand and Wrist pain
NET WT 1 OZ (28g)
NDC 72927-904-04
Visit www.jointflex.com for more information
* when applied as directed
** For skin conditioning
Sample not for retail sale.

-
INGREDIENTS AND APPEARANCE
JOINTFLEX
jointflex creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72927-904 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.2 g in 100 g Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ACETYLATED LANOLIN (UNII: 2X654GD19H) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER 1342 (UNII: 809Y72KV36) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) CHONDROITIN SULFATE (SHARK) (UNII: 2ZAJ1K50XH) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) GLUCOSAMINE SULFATE POTASSIUM CHLORIDE (UNII: 15VQ11I66N) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROXYLATED LANOLIN (UNII: EOI0B9800C) HYPROMELLOSES (UNII: 3NXW29V3WO) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) METHYL GLUCETH-20 (UNII: J3QD0LD11P) METHYL GLUCOSE SESQUISTEARATE (UNII: V1YW10H14D) PANTHENOL (UNII: WV9CM0O67Z) PEPPERMINT OIL (UNII: AV092KU4JH) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHARK CARTILAGE (UNII: D2YCN1I522) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72927-904-01 1 in 1 CARTON 08/02/2019 1 114 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:72927-904-03 1 in 1 CARTON 08/02/2019 2 28 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:72927-904-04 1 in 1 CARTON 08/02/2019 3 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/02/2019 Labeler - Strides Consumer LLC (116975770) Establishment Name Address ID/FEI Business Operations Beltapharm SPA 429236789 MANUFACTURE(72927-904) , PACK(72927-904)