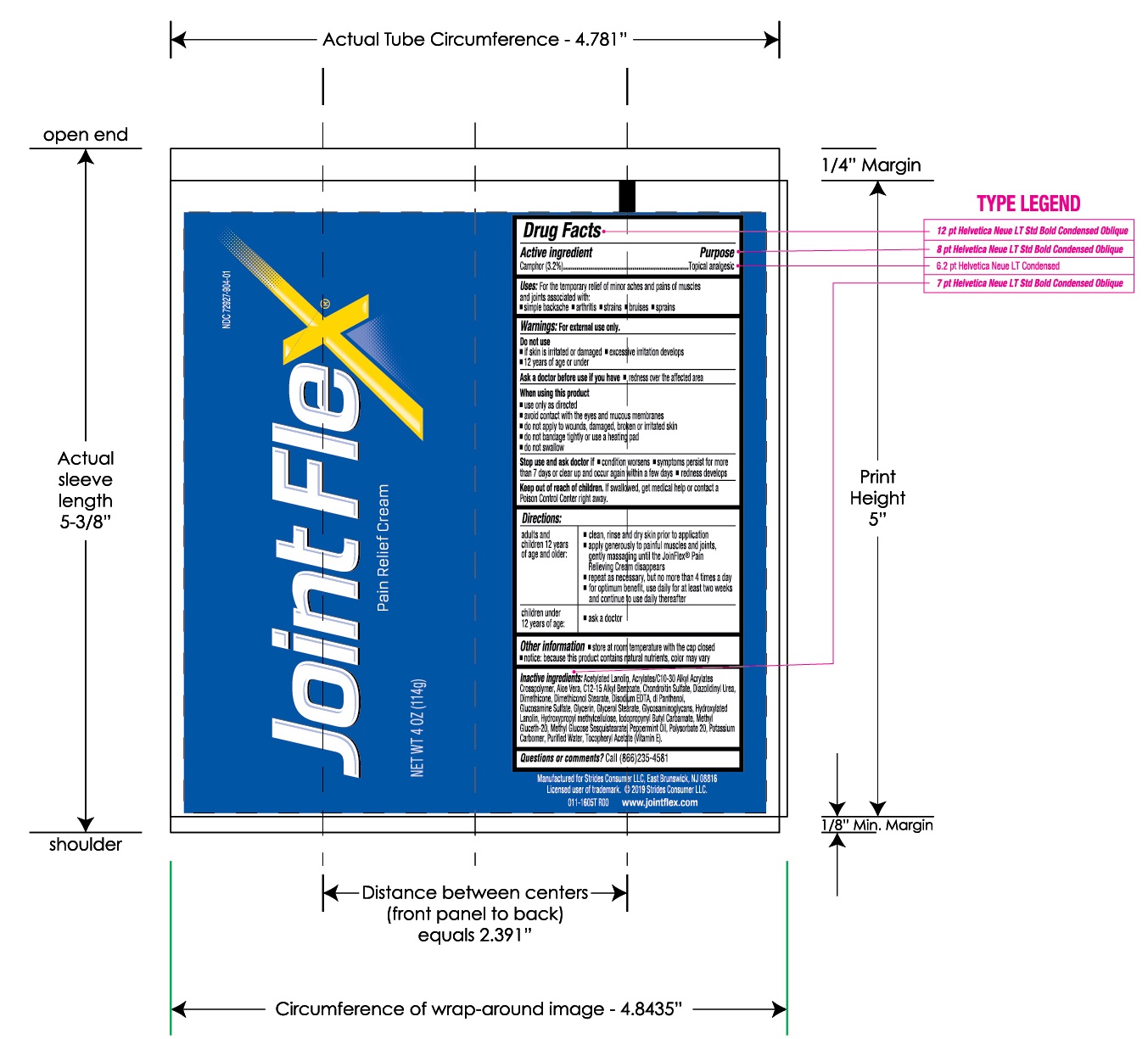
Uses:
For the temporary relief of minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
When using this product
- use only as directed
- avoid contact with the eyes and mucous membranes
- do not apply to wounds, damaged, broken or irritated skin
- do not bandage tightly or use a heating pad
- do not swallow
Stop use and ask doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
| adults and children 12 years of age and older: |
|
| children under 12 years of age: |
|
Other information
- store at room temperature with the cap closed
- notice: because this product contains natural nutrients, color may vary
Inactive ingredients:
Acetylated Lanolin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Vera, C12-15 Alkyl Benzoate, Chondroitin Sulfate, Diazolidinyl Urea, Dimethicone, Dimethiconol Stearate, Disodium EDTA, dl Panthenol, Glucosamine Sulfate, Glycerin, Glycerol Stearate, Glycosaminoglycans, Hydroxylated Lanolin, Hydroxypropyl methycellulose, Iodopropynyl Butylcarbamate, Methyl Gluceth-20, Methyl Glucose Sesquistearate, Peppermint Oil, Polysorbate 20, Potassium Carbomer, Purified Water, Tocopheryl Acetate (Vitamin E).
Questions and comments?
Call (866) 235-4581 or or email stridesconsumer@emersongroup.com
Manufactured for
Strides Consumer LLC,
East Brunswick, NJ 08816
†Results based on a 2021 Healthcare Provider survey conducted by InStep Health as part of a point-of-care marketing campaign sponsored by Strides Consumer LLC.
Manufactured for Strides Consumer LLC, East Brunswick, NJ 08816
Licensed user of trademarks. © 2022 Strides Consumer LLC
www.jointflex.com
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
8 Week Challenge for pain relief
Effectively Reduces Joint Pain
#1 CLINICALLY RECOMMENDED† JOINT PAIN CREAM
JointFlex®- Pain Relief Cream
Immediate & Long Lasting Relief*
Contains Glucosamine & Chondroitin**
No stain/ No Grease/ Pleasant Scent
DEEP PENETRATING Fusome® Technology
Quick & effective penetration
Targeted pain relief
Long lasting relief when used daily
Flex Your Freedom™
Relieves Back, Neck, Shoulder, Knees, Elbow, Foot, Ankle, Leg Hand and Wrist pain
NET WT 4 OZ (114g)
NDC 72927-904-01
Visit www.jointflex.com for more information
* when applied as directed
** For skin conditioning
011-1605C-R03

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
8 Week Challenge for pain relief
Effectively Reduces Joint Pain
JointFlex®- Pain Relief Cream
Immediate & Long Lasting Relief*
Contains Glucosamine & Chondroitin**
No stain/ No Grease/ Pleasant Scent
DEEP PENETRATING Fusome® Technology
Quick & effective penetration
Targeted pain relief
Long lasting relief when used daily
Flex Your Freedom™
Relieves Back, Neck, Shoulder, Knees, Elbow, Foot, Ankle, Leg Hand and Wrist pain
NET WT 1 OZ (28g)
NDC 72927-904-03
Visit www.jointflex.com for more information
* when applied as directed
** For skin conditioning
011-1606C-R01


8 Week Challenge for pain relief
Effectively Reduces Joint Pain
JointFlex®- Pain Relief Cream
Immediate & Long Lasting Relief*
Contains Glucosamine & Chondroitin**
No stain/ No Grease/ Pleasant Scent
DEEP PENETRATING Fusome® Technology
Quick & effective penetration
Targeted pain relief
Long lasting relief when used daily
Flex Your Freedom™
Relieves Back, Neck, Shoulder, Knees, Elbow, Foot, Ankle, Leg Hand and Wrist pain
NET WT 1 OZ (28g)
NDC 72927-904-04
Visit www.jointflex.com for more information
* when applied as directed
** For skin conditioning
Sample not for retail sale.
