Label: TETROXY 25- oxytetracycline hcl powder
- NDC Code(s): 61133-5025-1
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Tetroxy® 25
(oxytetracycline HCl)
Soluble Powder
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
A broad spectrum ANTOBIOTIC
FOR CONTROL AND TREATMENT OF SPECIFIC DISEASES
IN POULTRY, CATTLE, SHEEP, SWINE AND HONEY BEES.
This packet contains 10 grams of oxytetracycline hydrochloride
For oral use in poultry, cattle, sheep, swine and honey bees.
FOR USE IN DRINKING WATER ONLY
NOT FOR USE IN LIQUID FEED SUPPLEMENTS
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Restricted Drug (California) - Use Only as DirectedANADA 200-146, Approved by FDA
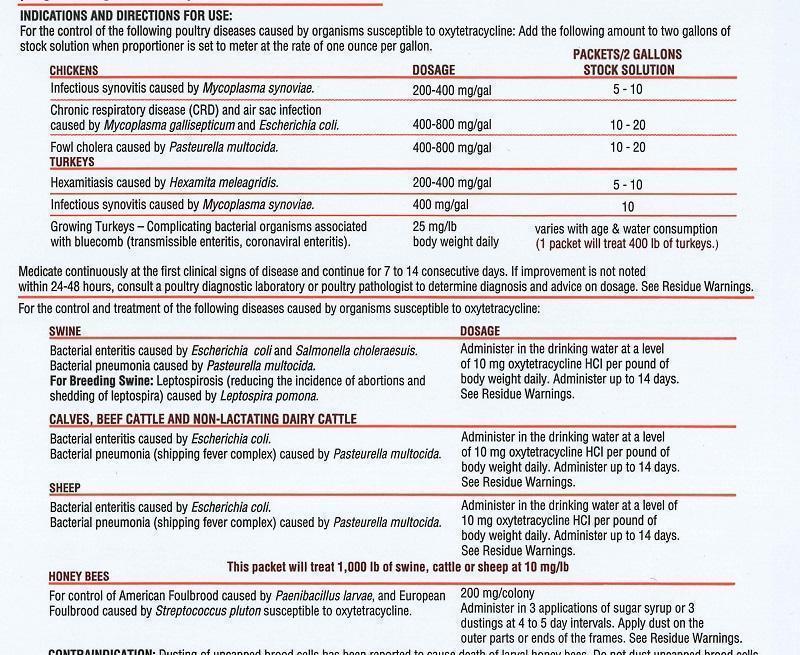
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
PRECAUTIONS: Use as sole source of oxytetracycline. Prepare fresh solutions every 24 hours.
Special Note: The concentration of drug required in medicated water must be adequate to compensate for variation in the age of the animal, feedconsumption rate and the environmental temperature and humidity, each of which affects water consumption.
-
RESIDUE WARNING
RESIDUE WARNING: Do not administer turkeys, cattle or sheep within 5 days of slaughter. Zero-day slaughter withdrawal in swine. Do not administer to chickens or turkeys producing eggs for human consumption. Do not administer this product with milk replacers. Adminster 1 hour before or 2 hours after feeding milk or milk replacers. A milk discard period has not been estblished for this product in lactating dairy cattle. Do not use in female dairy cattle 20 months of age or older. For honey bees, the drug should be fed early in the spring or fall and consumed by the bees before main honey flow begins to avoid contamination of production honey. Remove at least 6 weeks prior to main honey flow.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TETROXY 25
oxytetracycline hcl powderProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-5025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYTETRACYCLINE HYDROCHLORIDE (UNII: 4U7K4N52ZM) (OXYTETRACYCLINE ANHYDROUS - UNII:SLF0D9077S) OXYTETRACYCLINE HYDROCHLORIDE 10 g in 181.5 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-5025-1 181.5 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200146 09/28/2017 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture Establishment Name Address ID/FEI Business Operations Yangzhou Liberty Pharmaceutical Co., Ltd. 529588375 api manufacture