Tetroxy® 25
(oxytetracycline HCl)
Soluble Powder
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
A broad spectrum ANTOBIOTIC
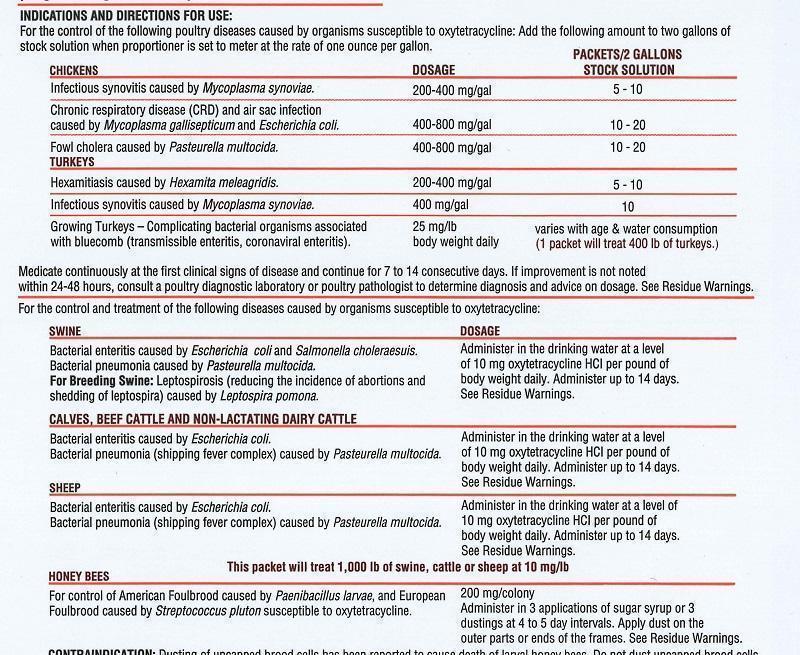
FOR CONTROL AND TREATMENT OF SPECIFIC DISEASES
IN POULTRY, CATTLE, SHEEP, SWINE AND HONEY BEES.
This packet contains 10 grams of oxytetracycline hydrochloride
For oral use in poultry, cattle, sheep, swine and honey bees.
FOR USE IN DRINKING WATER ONLY
NOT FOR USE IN LIQUID FEED SUPPLEMENTS
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Restricted Drug (California) - Use Only as Directed
ANADA 200-146, Approved by FDA
CONTRAINDICATION: Dusting of uncapped brood cells has been reported to cause death of larval honey bees. Do not dust uncapped brood cells.
PRECAUTIONS: Use as sole source of oxytetracycline. Prepare fresh solutions every 24 hours.
Special Note: The concentration of drug required in medicated water must be adequate to compensate for variation in the age of the animal, feedconsumption rate and the environmental temperature and humidity, each of which affects water consumption.
RESIDUE WARNING: Do not administer turkeys, cattle or sheep within 5 days of slaughter. Zero-day slaughter withdrawal in swine. Do not administer to chickens or turkeys producing eggs for human consumption. Do not administer this product with milk replacers. Adminster 1 hour before or 2 hours after feeding milk or milk replacers. A milk discard period has not been estblished for this product in lactating dairy cattle. Do not use in female dairy cattle 20 months of age or older. For honey bees, the drug should be fed early in the spring or fall and consumed by the bees before main honey flow begins to avoid contamination of production honey. Remove at least 6 weeks prior to main honey flow.