Label: ANTIFUNGAL CREAM- antifungal cream
- NDC Code(s): 68001-475-45, 68001-475-46, 68001-475-47
- Packager: BluePoint Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use:
- on children under 2 years of age unless directed by a doctor.
When using this product
- Avoid contact with eyes.
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 2 weeks when used for the treatment of jock itch.
- there is no improvement within 4 weeks when used for athlete's foot or ringworm.
- Keep Out Of Reach Of Children
-
Directions
- Clean the affected area and dry thoroughly. Apply a thin layer of cream over affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product.
- For athlete's foot: Pay special attention to spaces between the toes: wear well fitting, ventilated shoes, and change shoes and socks at least once daily.
- DOSAGE & ADMINISTRATION
- Other Information
- INACTIVE INGREDIENTS
- Questions?
- PRINCIPAL DISPLAY PANEL
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ANTIFUNGAL CREAM
antifungal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68001-475 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) WHITE PETROLATUM (UNII: B6E5W8RQJ4) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) MINERAL OIL (UNII: T5L8T28FGP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-475-47 1 in 1 CARTON 11/25/2020 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68001-475-46 1 in 1 CARTON 11/25/2020 06/14/2021 2 15 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:68001-475-45 1 in 1 CARTON 11/25/2020 3 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 11/25/2020 Labeler - BluePoint Laboratories (985523874) Establishment Name Address ID/FEI Business Operations Gopaldas Visram & Co., Ltd 858030888 manufacture(68001-475)


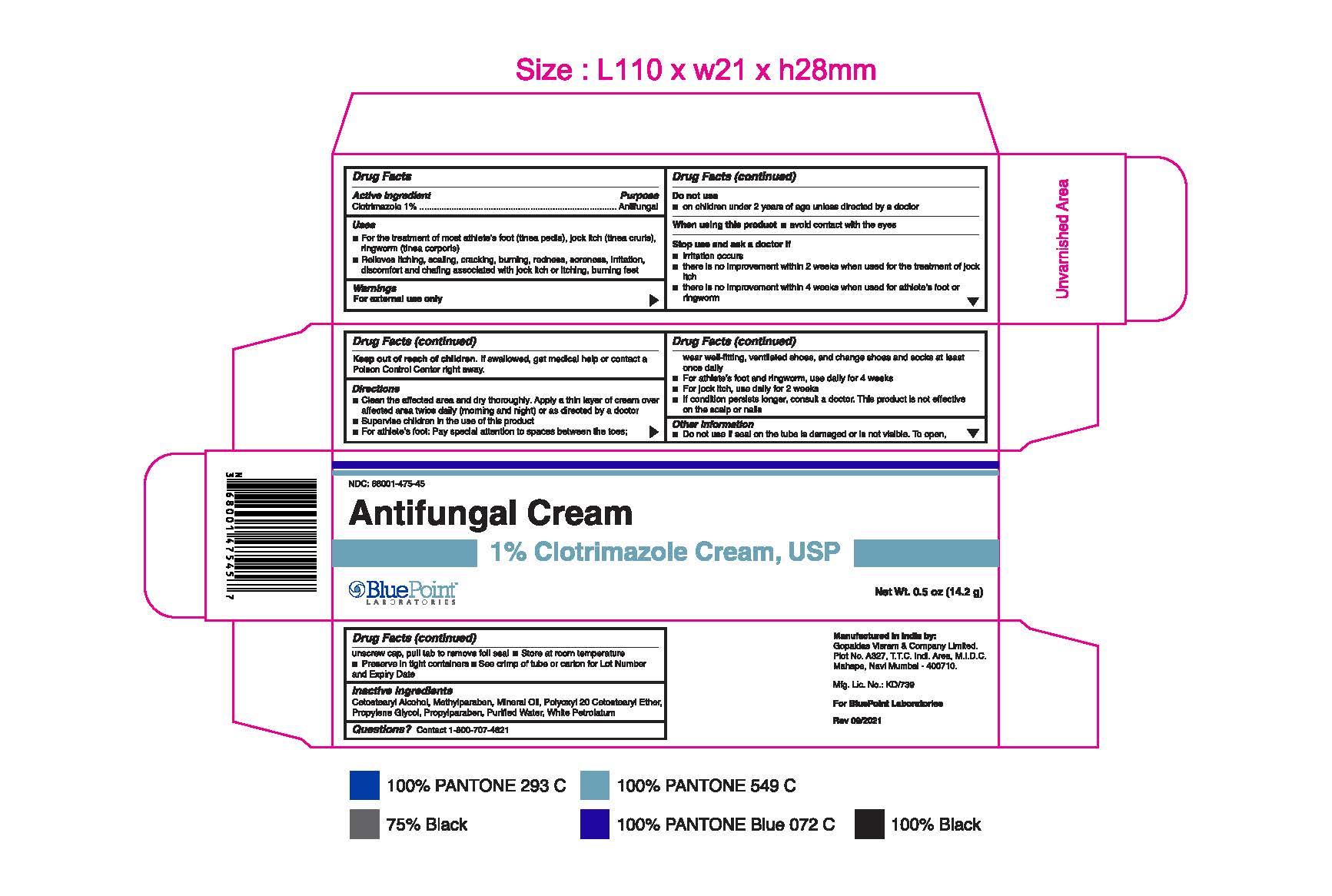
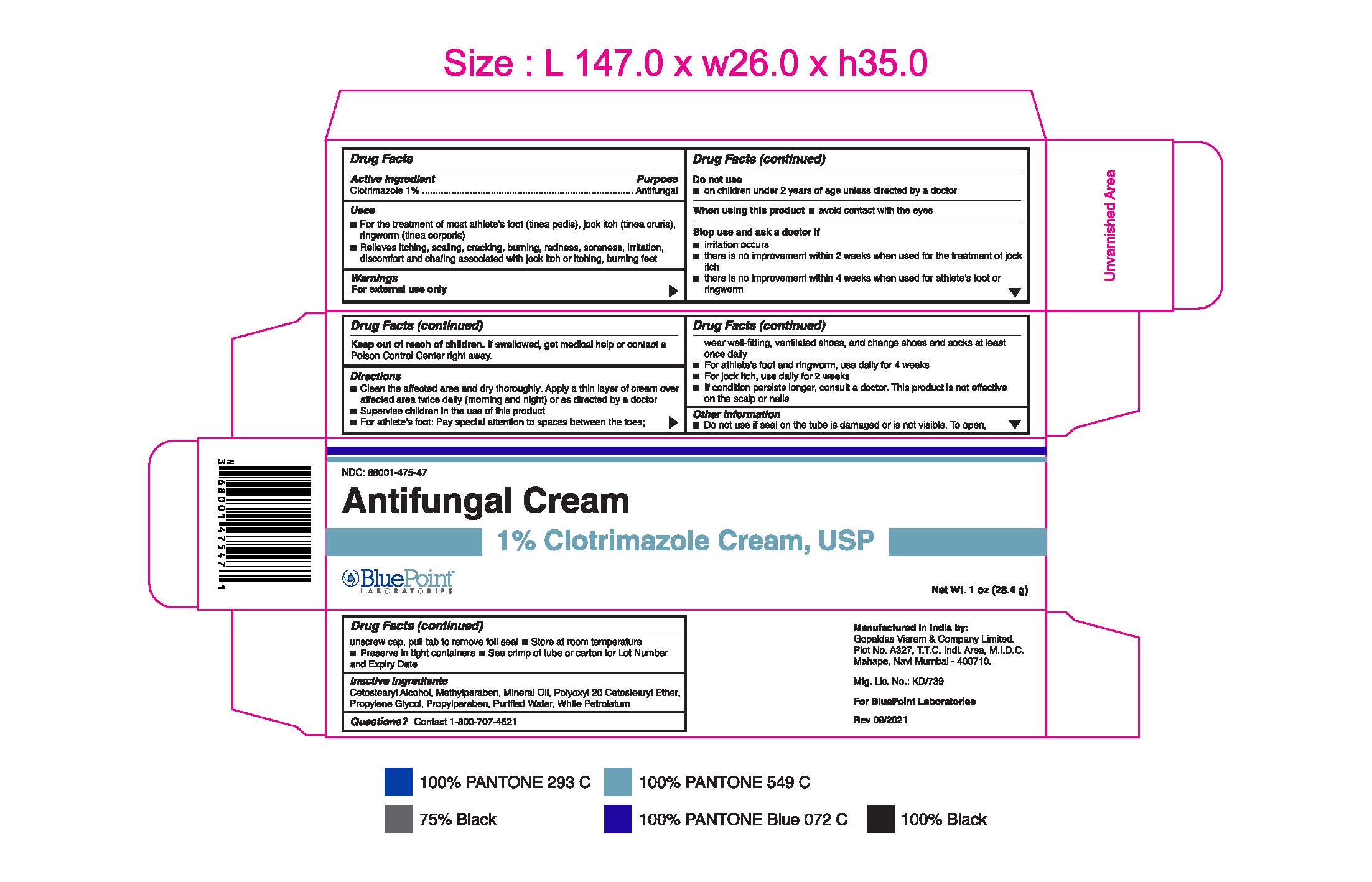


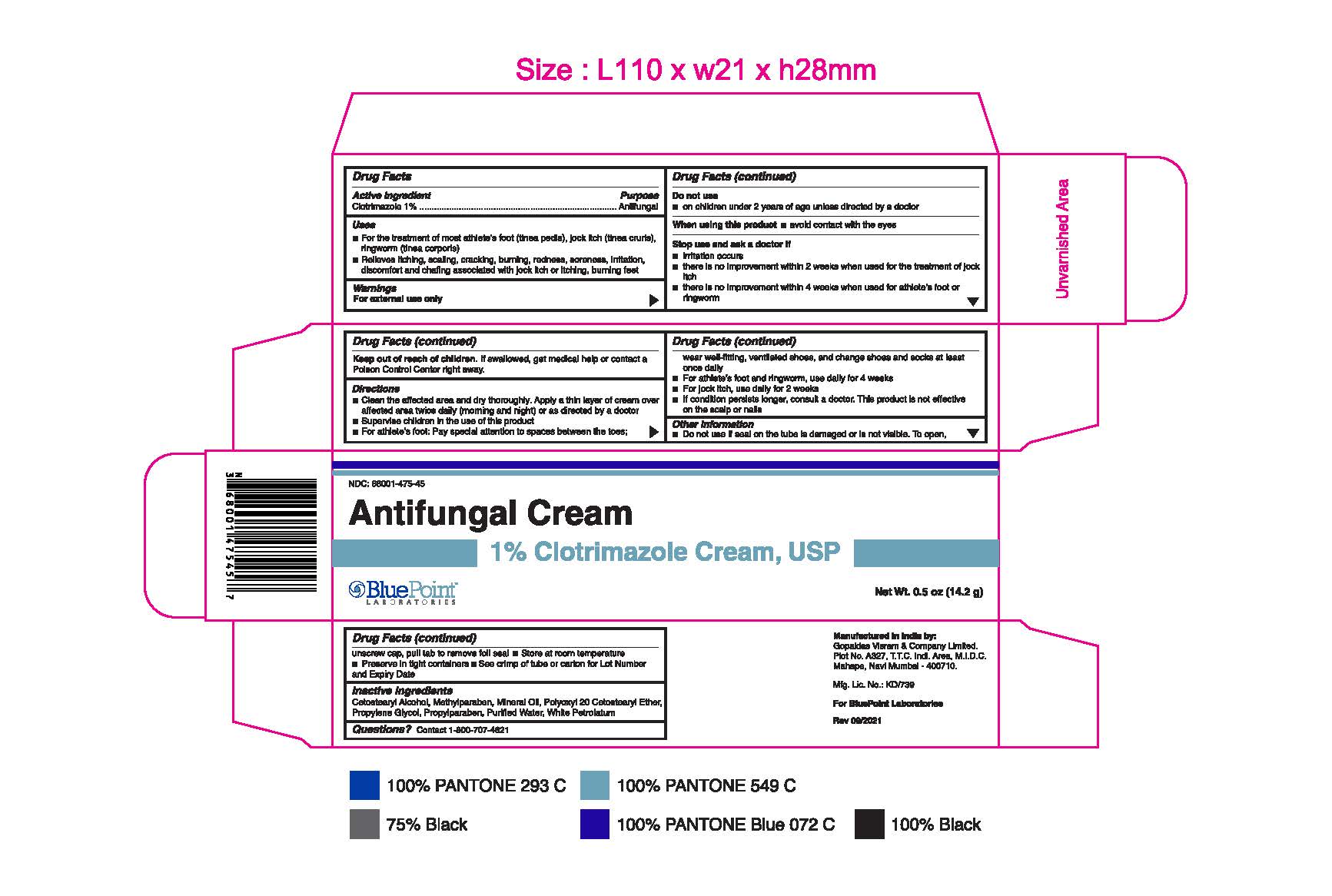
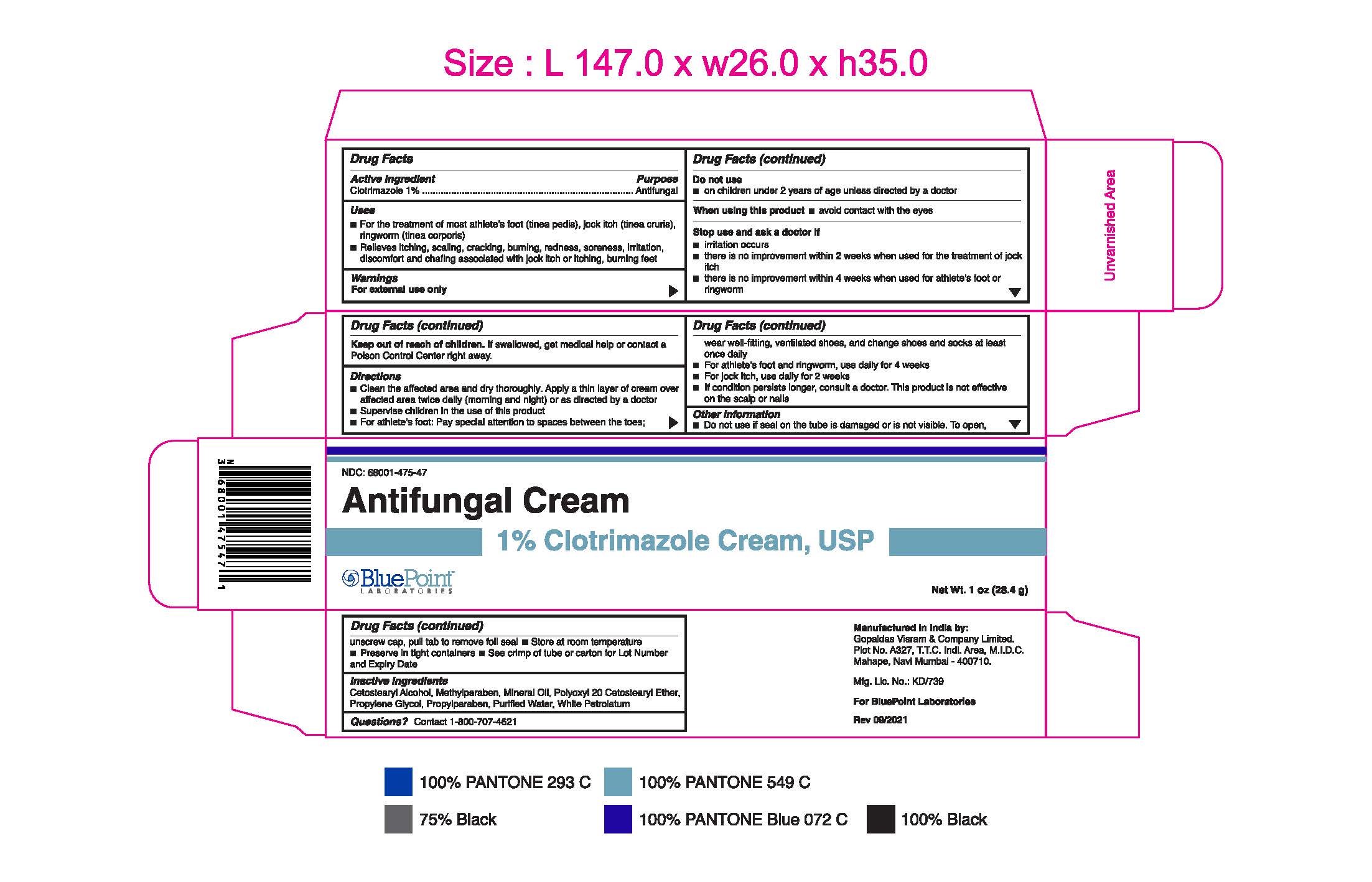 Clotrimazole 1% 1oz 68001-475-47
Clotrimazole 1% 1oz 68001-475-47
