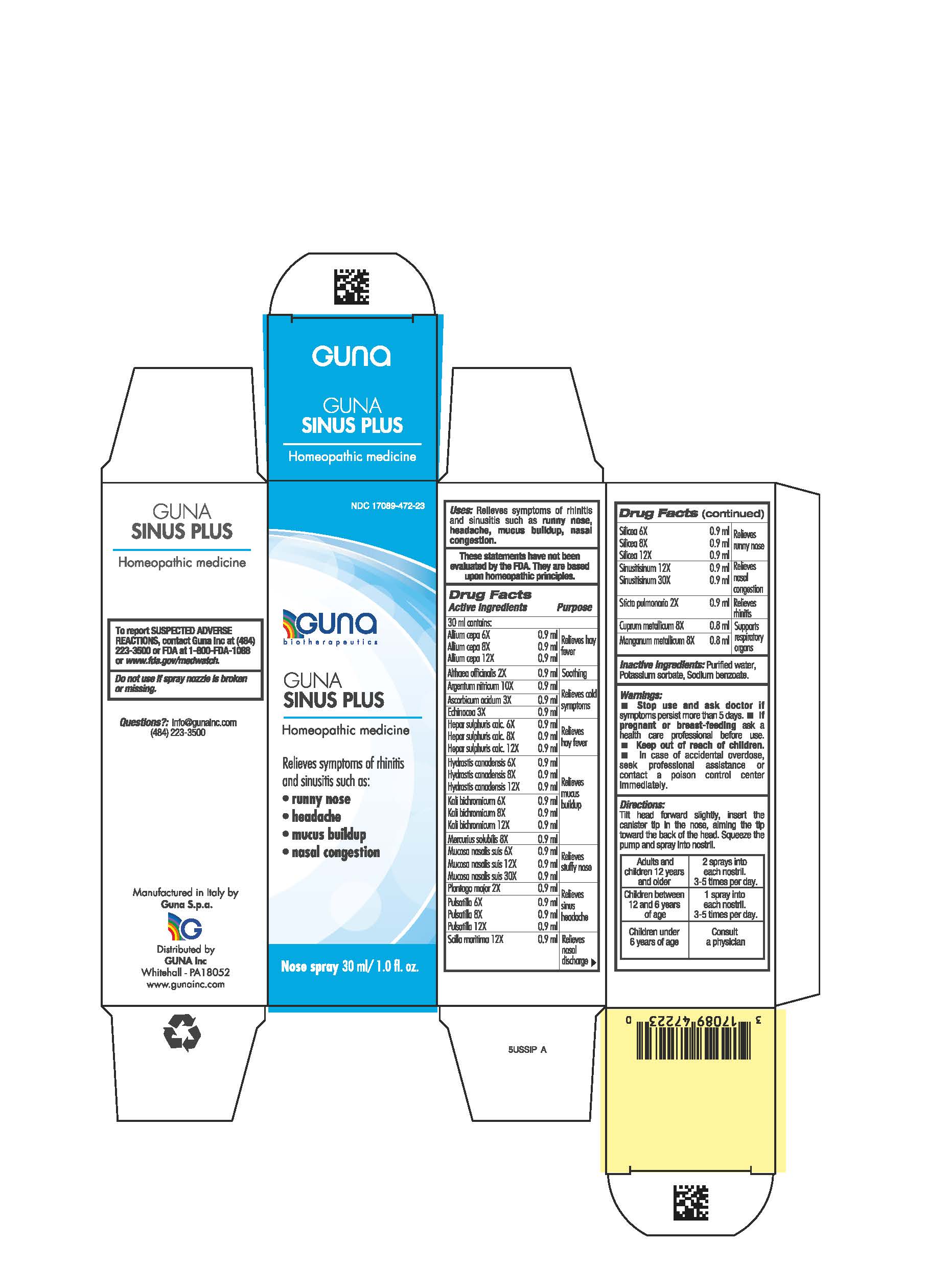
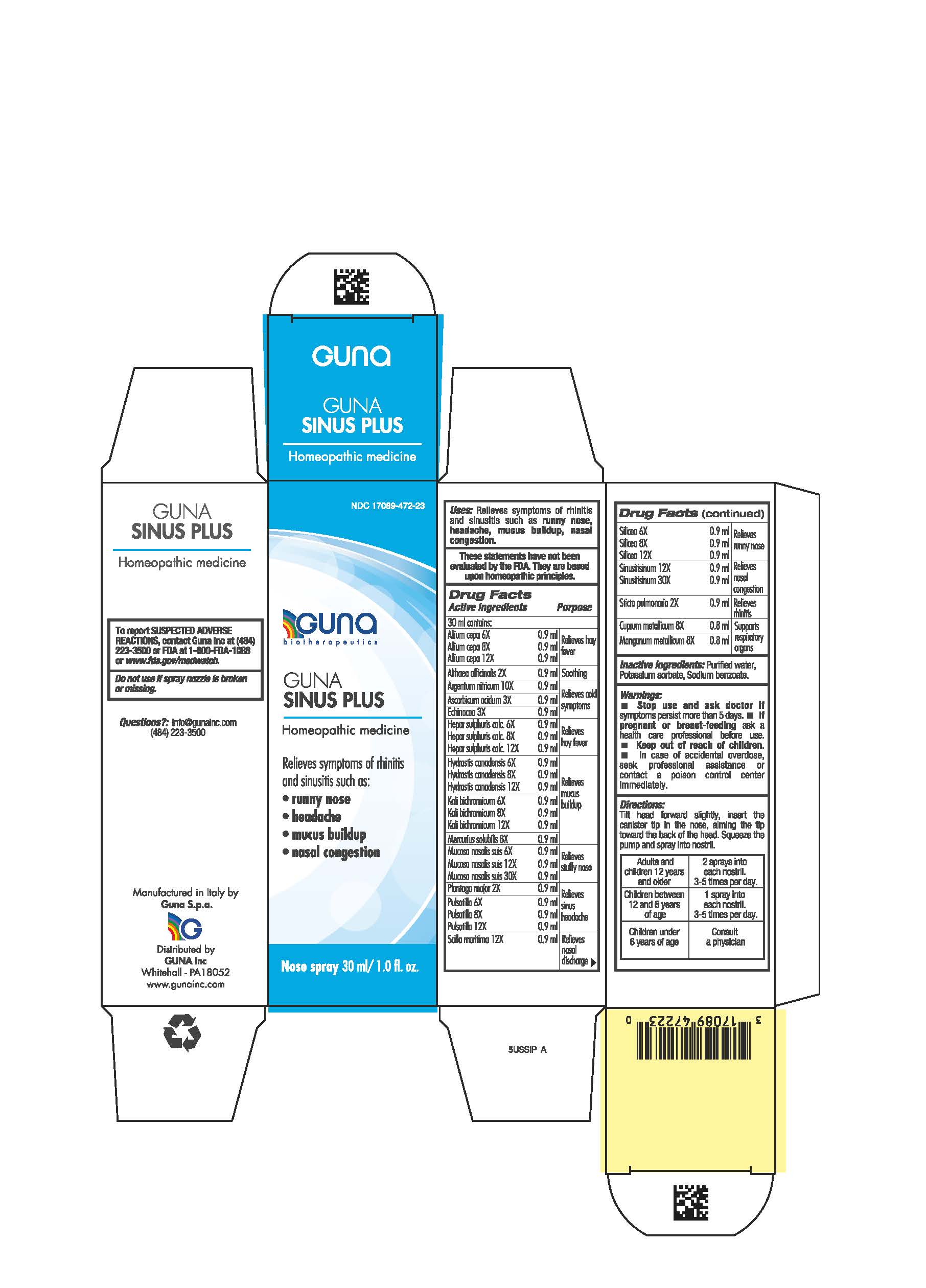
Label: GUNA SINUS PLUS- althaea officinalis leaf - calcium sulfide - copper - drimia maritima bulb - echinacea angustifolia - hydrastis canadensis whole - lobaria pulmonaria - manganese - mercuric sulfide - onion - plantago major whole - potassium dichromate - pulsatilla vulgaris - silicon dioxide - silver nitrate - sinusitisinum - sus scrofa nasal mucosa - spray
- NDC Code(s): 17089-472-23
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 17, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DIRECTIONS
-
ACTIVE INGREDIENTS/PURPOSE
- ALLIUM CEPA 6X, 8X, 12X Relieves hay fever
- ALTHEA OFFICINALIS 2X Soothing
- ARGENTUM NITRICUM 10X Relieves cold symptoms
- ASCORBIC ACIDUM 3X Relieves cold symptoms
- ECHINACEA 3X Relieves cold symptoms
- HEPAR SULPHURIS CALCAREUM 6X, 8X, 12X Relieves hay fever
- HYDRASTIS CANADENSIS 6X, 8X, 12X Relieves mucus buildup
- KALI BICHROMICUM 6X, 8X, 12X Relieves mucus buildup
- MERCURIUS SOLUBILIS 8X Relieves mucus buildup
- MUCOSA NASALIS SUIS 6X, 12X, 30X Relieves stuffy nose
- PLANTAGO MAJOR 2X Relieves sinus headache
- PULSATILLA 6X, 8X, 12X Relieves sinus headache
- SCILLA MARITIMA 12X Relieves nasal discharge
- SILICEA 6X, 8X, 12X Relieves runny nose
- SINUSITISINUM 12X, 30X Relieves nasal congestion
- STICTA PULMONARIA 2X Relieves rhinitis
- CUPRUM METALLICUM 8X Supports respiratory organs
- MANGANUM METALLICUM 8X Supports respiratory organs
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- WARNINGS
- PREGNANCY
- WARNINGS
- USES
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA SINUS PLUS
althaea officinalis leaf - calcium sulfide - copper - drimia maritima bulb - echinacea angustifolia - hydrastis canadensis whole - lobaria pulmonaria - manganese - mercuric sulfide - onion - plantago major whole - potassium dichromate - pulsatilla vulgaris - silicon dioxide - silver nitrate - sinusitisinum - sus scrofa nasal mucosa - sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-472 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 30 mL ALTHAEA OFFICINALIS LEAF (UNII: E2QQV92338) (ALTHAEA OFFICINALIS LEAF - UNII:E2QQV92338) ALTHAEA OFFICINALIS LEAF 2 [hp_X] in 30 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 30 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 3 [hp_X] in 30 mL HYDRASTIS CANADENSIS WHOLE (UNII: R763EBH88T) (HYDRASTIS CANADENSIS WHOLE - UNII:R763EBH88T) HYDRASTIS CANADENSIS WHOLE 6 [hp_X] in 30 mL LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 2 [hp_X] in 30 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 8 [hp_X] in 30 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 30 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 6 [hp_X] in 30 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 6 [hp_X] in 30 mL MANGANESE (UNII: 42Z2K6ZL8P) (MANGANESE - UNII:42Z2K6ZL8P) MANGANESE 8 [hp_X] in 30 mL MERCURIC SULFIDE (UNII: ZI0T668SF1) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC SULFIDE 8 [hp_X] in 30 mL SUS SCROFA NASAL MUCOSA (UNII: ID3Z1X61WY) (SUS SCROFA NASAL MUCOSA - UNII:ID3Z1X61WY) SUS SCROFA NASAL MUCOSA 6 [hp_X] in 30 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_X] in 30 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 6 [hp_X] in 30 mL SINUSITISINUM (UNII: B575563DM5) (SINUSITISINUM - UNII:B575563DM5) SINUSITISINUM 12 [hp_X] in 30 mL PLANTAGO MAJOR WHOLE (UNII: W2469WNO6U) (PLANTAGO MAJOR WHOLE - UNII:W2469WNO6U) PLANTAGO MAJOR WHOLE 2 [hp_X] in 30 mL DRIMIA MARITIMA BULB (UNII: 3629601H5D) (DRIMIA MARITIMA BULB - UNII:3629601H5D) DRIMIA MARITIMA BULB 12 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 0.03 mL in 30 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.03 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-472-23 1 in 1 BOX 02/17/2021 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/17/2021 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-472)