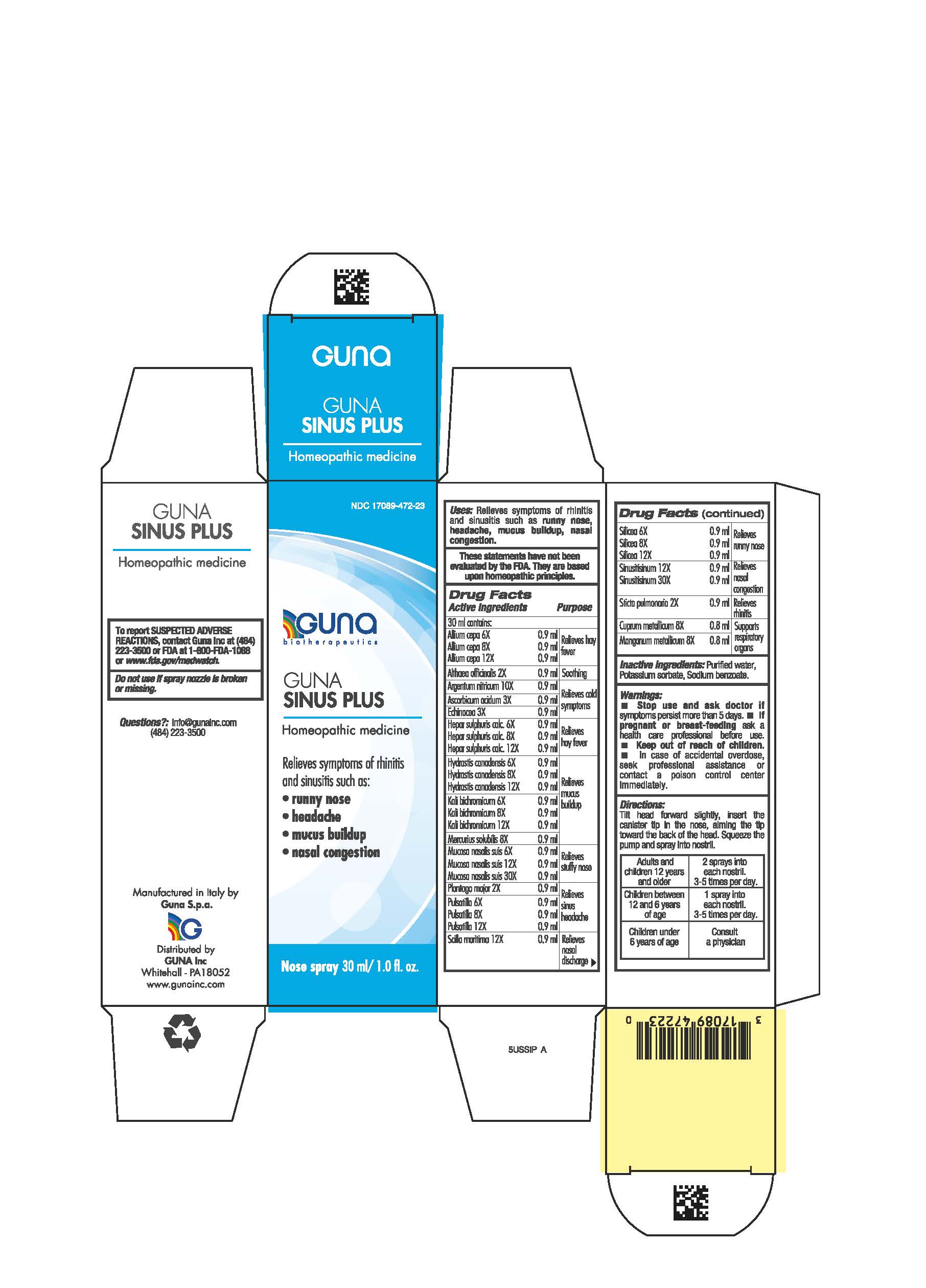
DIRECTIONS
Adults and children 12 years and older 2 sprays to each nostril, 3-5 times per day
Children between 12 years and 6 years of age 1 spray to each nostril, 3-5 times per day
Children under 6 years consult a physician
ACTIVE INGREDIENTS/PURPOSE
- ALLIUM CEPA 6X, 8X, 12X Relieves hay fever
- ALTHEA OFFICINALIS 2X Soothing
- ARGENTUM NITRICUM 10X Relieves cold symptoms
- ASCORBIC ACIDUM 3X Relieves cold symptoms
- ECHINACEA 3X Relieves cold symptoms
- HEPAR SULPHURIS CALCAREUM 6X, 8X, 12X Relieves hay fever
- HYDRASTIS CANADENSIS 6X, 8X, 12X Relieves mucus buildup
- KALI BICHROMICUM 6X, 8X, 12X Relieves mucus buildup
- MERCURIUS SOLUBILIS 8X Relieves mucus buildup
- MUCOSA NASALIS SUIS 6X, 12X, 30X Relieves stuffy nose
- PLANTAGO MAJOR 2X Relieves sinus headache
- PULSATILLA 6X, 8X, 12X Relieves sinus headache
- SCILLA MARITIMA 12X Relieves nasal discharge
- SILICEA 6X, 8X, 12X Relieves runny nose
- SINUSITISINUM 12X, 30X Relieves nasal congestion
- STICTA PULMONARIA 2X Relieves rhinitis
- CUPRUM METALLICUM 8X Supports respiratory organs
- MANGANUM METALLICUM 8X Supports respiratory organs
Directions:
tilt head forward slightly, insert the canister tip in the nose, aiming the tip toward the back of the head. Squeeze the pump and spray intro nostril.
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, seek professional assistance or contact a Poison Control Center immediately.