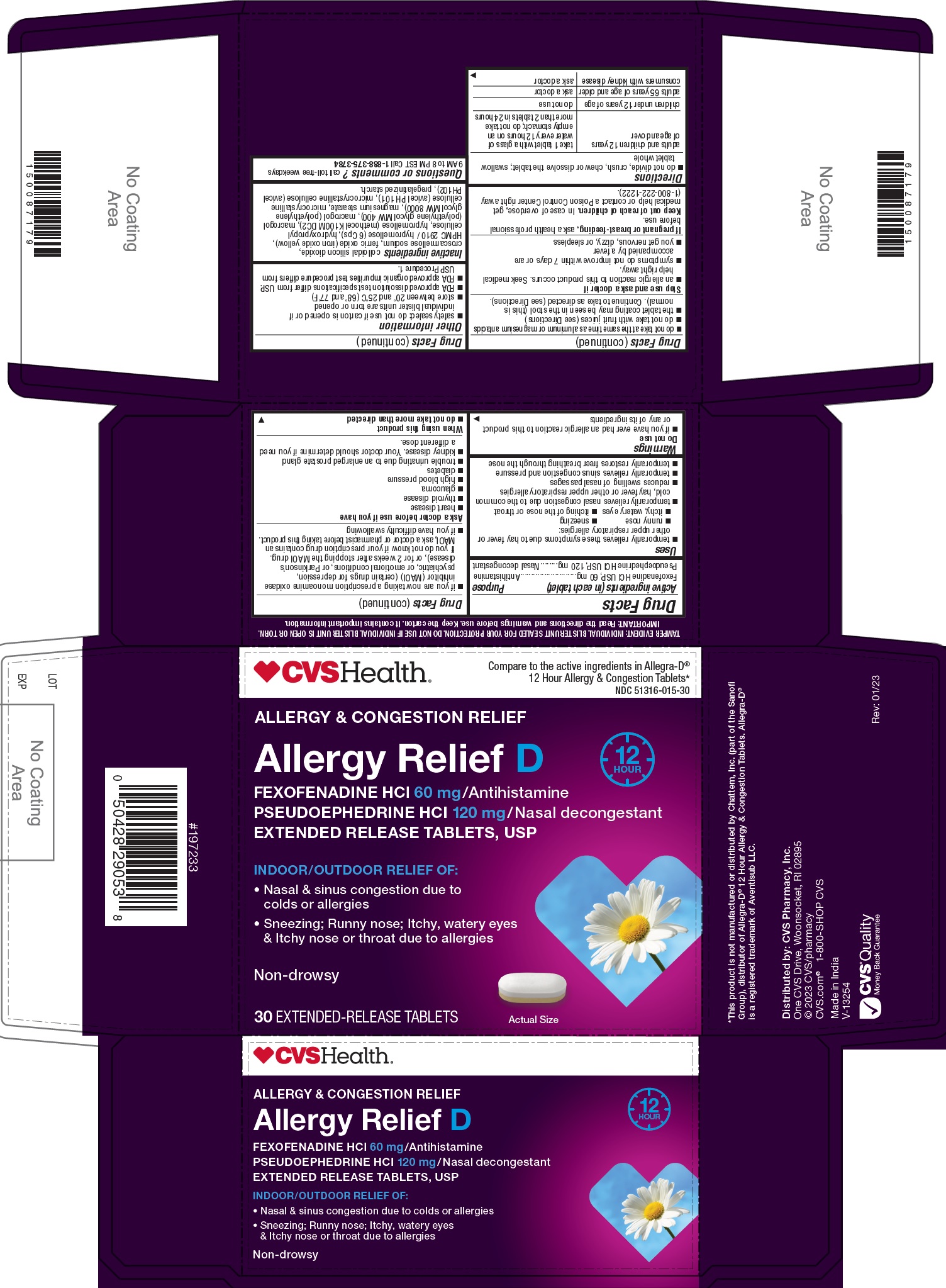
Label: FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE tablet, extended release
- NDC Code(s): 51316-015-20, 51316-015-30
- Packager: CVS Health Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
- Purpose
-
Use(s)
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relives nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
-
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- the tablet coating may be seen in the stool (this is normal). Continue to take as directed (see Directions).
-
Directions
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
adults and children 12 years of age and over take 1 tablet with a glass of water every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor -
Other information
- safety sealed: do not use if carton is opened or if individual blister units are torn or opened
- store between 20° to 25°C (68° to 77°F) store between 20° to 25°C (68° to 77°F)
- FDA approved dissolution test specifications differ from USP.
- FDA approved organic impurities test procedure differs from USP Procedure 1.
-
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, ferric oxide (iron oxide yellow), HPMC 2910 / hypromellose (6 Cps), hydroxypropyl cellulose, hypromellose (methocel K100M DC2), macrogol (polyethylene glycol MW 400), macrogol (polyethylene glycol MW 8000), magnesium stearate, microcrystalline cellulose (avicel PH 101), microcrystalline cellulose (avicel PH102), pregelatinized starch.
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-015 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) Croscarmellose Sodium (UNII: M28OL1HH48) Ferric Oxide Yellow (UNII: EX438O2MRT) Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Stearate (UNII: 70097M6I30) Polyethylene Glycol 400 (UNII: B697894SGQ) Hypromellose 2208 (100000 Mpa.S) (UNII: VM7F0B23ZI) HYDROXYPROPYL CELLULOSE (45000 WAMW) (UNII: 8VAB711C5E) Hypromellose 2910 (6 Mpa.S) (UNII: 0WZ8WG20P6) Polyethylene Glycol 8000 (UNII: Q662QK8M3B) Starch, Corn (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) Product Characteristics Color WHITE (one white to off-white color layer and other light yellow to yellow color) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-015-20 4 in 1 CARTON 03/17/2023 1 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:51316-015-30 6 in 1 CARTON 03/17/2023 2 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215434 06/03/2022 Labeler - CVS Health Corp (062312574)