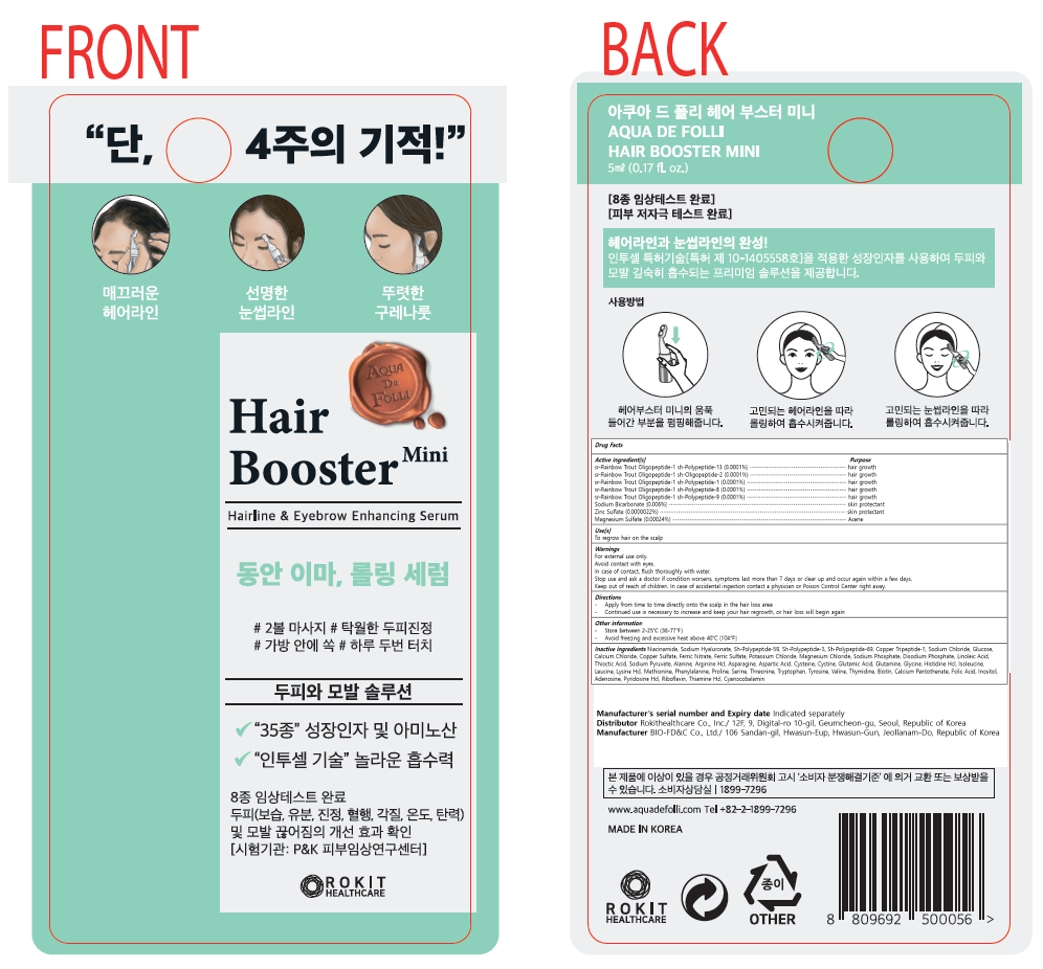
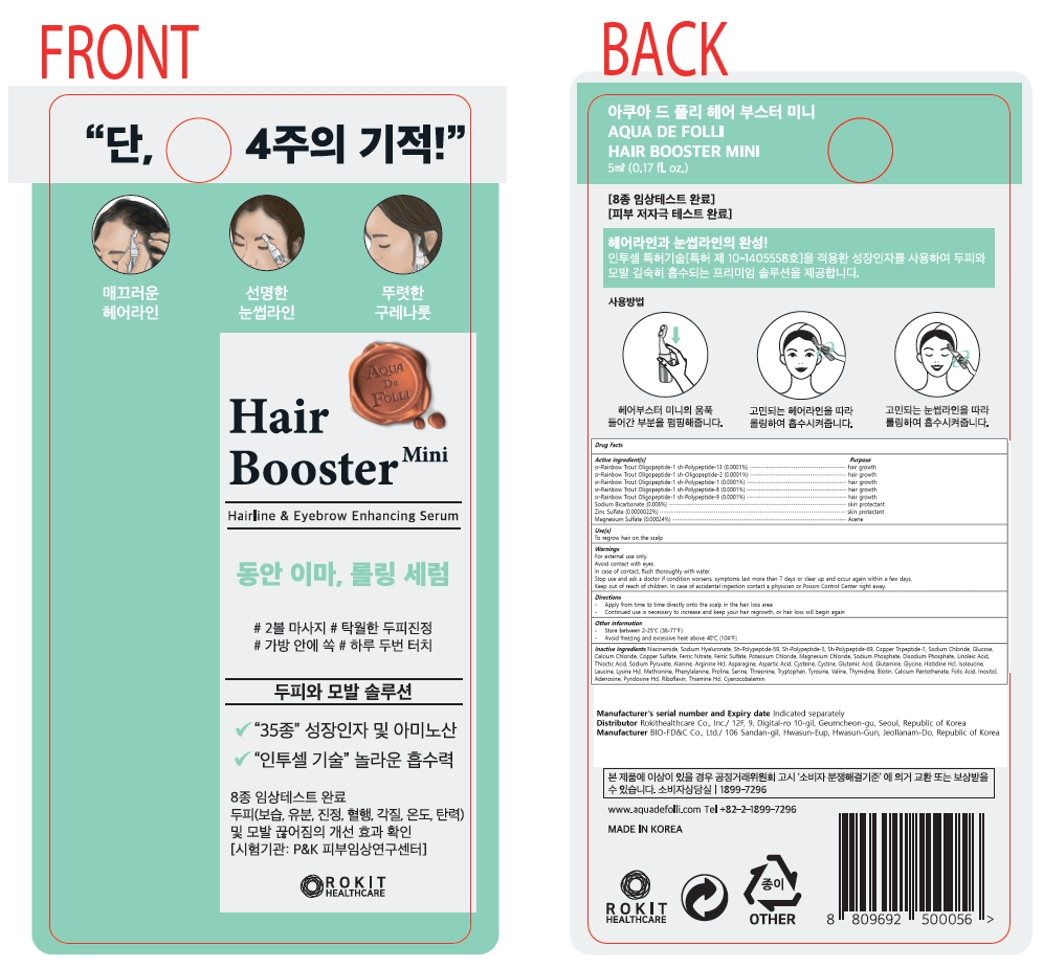
Label: AQUA DE FOLLI HAIR BOOSTER MINI- sr-rainbow trout oligopeptide-1 sh-polypeptide-13, sr-rainbow trout oligopeptide-1 sh-oligopeptide-2, sr-rainbow trout oligopeptide-1 sh-polypeptide-1, sr-rainbow trout oligopeptide-1 sh-polypeptide-8, sr-rainbow trout oligopeptide-1 sh-polypeptide-9, sodium bicarbonate, zinc sulfate, magnesium sulfate liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 80592-203-01 - Packager: ROKIT HEALTHCARE Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 5, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient[s]
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-13 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Oligopeptide-2 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-1 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-8 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-9 (0.0001%)
Sodium Bicarbonate (0.006%)
Zinc Sulfate (0.0000022%)
Magnesium Sulfate (0.00024%)
- Purpose
- Use[s]
- Warnings
- Warnings
- Warnings
- Directions
- Other information
-
Inactive ingredients
Niacinamide, Sodium Hyaluronate, Sh-Polypeptide-59, Sh-Polypeptide-3, Sh-Polypeptide-69, Copper Tripeptide-1, Sodium Chloride, Glucose, Calcium Chloride, Copper Sulfate, Ferric Nitrate, Ferric Sulfate, Potassium Chloride, Magnesium Chloride, Sodium Phosphate, Disodium Phosphate, Linoleic Acid, Thioctic Acid, Sodium Pyruvate, Alanine, Arginine Hcl, Asparagine, Aspartic Acid, Cysteine, Cystine, Glutamic Acid, Glutamine, Glycine, Histidine Hcl, Isoleucine, Leucine, Lysine Hcl, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, Valine, Thymidine, Biotin, Calcium Pantothenate, Folic Acid, Inositol, Adenosine, Pyridoxine Hcl, Riboflavin, Thiamine Hcl, Cyanocobalamin, Coceth-7, PEG-40 Hydrogenated Castor Oil, PPG-1-PEG-9 Lauryl Glycol Ether
- Package Label
-
INGREDIENTS AND APPEARANCE
AQUA DE FOLLI HAIR BOOSTER MINI
sr-rainbow trout oligopeptide-1 sh-polypeptide-13, sr-rainbow trout oligopeptide-1 sh-oligopeptide-2, sr-rainbow trout oligopeptide-1 sh-polypeptide-1, sr-rainbow trout oligopeptide-1 sh-polypeptide-8, sr-rainbow trout oligopeptide-1 sh-polypeptide-9, sodium bicarbonate, zinc sulfate, magnesium sulfate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80592-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC SULFATE (UNII: 89DS0H96TB) (ZINC CATION - UNII:13S1S8SF37) ZINC SULFATE 0.0000022 g in 100 mL SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 0.006 g in 100 mL MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) (SULFATE ION - UNII:7IS9N8KPMG) MAGNESIUM SULFATE, UNSPECIFIED 0.00024 g in 100 mL Inactive Ingredients Ingredient Name Strength ADENOSINE (UNII: K72T3FS567) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) RIBOFLAVIN (UNII: TLM2976OFR) CYANOCOBALAMIN (UNII: P6YC3EG204) COCETH-7 (UNII: 58Y261JLH5) GLYCINE (UNII: TE7660XO1C) WATER (UNII: 059QF0KO0R) ISOLEUCINE (UNII: 04Y7590D77) INOSITOL (UNII: 4L6452S749) SODIUM PHOSPHATE (UNII: SE337SVY37) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) LEUCINE (UNII: GMW67QNF9C) THREONINE (UNII: 2ZD004190S) TRYPTOPHAN (UNII: 8DUH1N11BX) TYROSINE (UNII: 42HK56048U) THYMIDINE (UNII: VC2W18DGKR) FOLIC ACID (UNII: 935E97BOY8) THIAMINE HYDROCHLORIDE (UNII: M572600E5P) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PPG-1-PEG-9 LAURYL GLYCOL ETHER (UNII: 5R8J43K25L) METHIONINE (UNII: AE28F7PNPL) ASPARAGINE (UNII: 5Z33R5TKO7) GLUTAMIC ACID (UNII: 3KX376GY7L) LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) PHENYLALANINE (UNII: 47E5O17Y3R) PROLINE (UNII: 9DLQ4CIU6V) SERINE (UNII: 452VLY9402) VALINE (UNII: HG18B9YRS7) BIOTIN (UNII: 6SO6U10H04) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) FIBROBLAST GROWTH FACTOR 7 (UNII: 9YXF283GP1) ALANINE (UNII: OF5P57N2ZX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) NIACINAMIDE (UNII: 25X51I8RD4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BECAPLERMIN (UNII: 1B56C968OA) PREZATIDE COPPER (UNII: 6BJQ43T1I9) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) FERRIC SULFATE (UNII: 3HWS7HF5XD) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) LINOLEIC ACID (UNII: 9KJL21T0QJ) THIOCTIC ACID (UNII: 73Y7P0K73Y) SODIUM PYRUVATE (UNII: POD38AIF08) ASPARTIC ACID (UNII: 30KYC7MIAI) CYSTEINE (UNII: K848JZ4886) CYSTINE (UNII: 48TCX9A1VT) GLUTAMINE (UNII: 0RH81L854J) FERRIC NITRATE (UNII: N8H8402XOB) HUMAN TYPE III COLLAGEN (UNII: 5D8UAE62VB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) CUPRIC SULFATE (UNII: LRX7AJ16DT) POTASSIUM CHLORIDE (UNII: 660YQ98I10) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80592-203-01 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/24/2020 Labeler - ROKIT HEALTHCARE Inc. (689004656) Registrant - ROKIT HEALTHCARE Inc. (689004656) Establishment Name Address ID/FEI Business Operations ROKIT HEALTHCARE Inc. 689004656 label(80592-203) Establishment Name Address ID/FEI Business Operations BIO-FD&C CO., LTD 694287377 manufacture(80592-203)