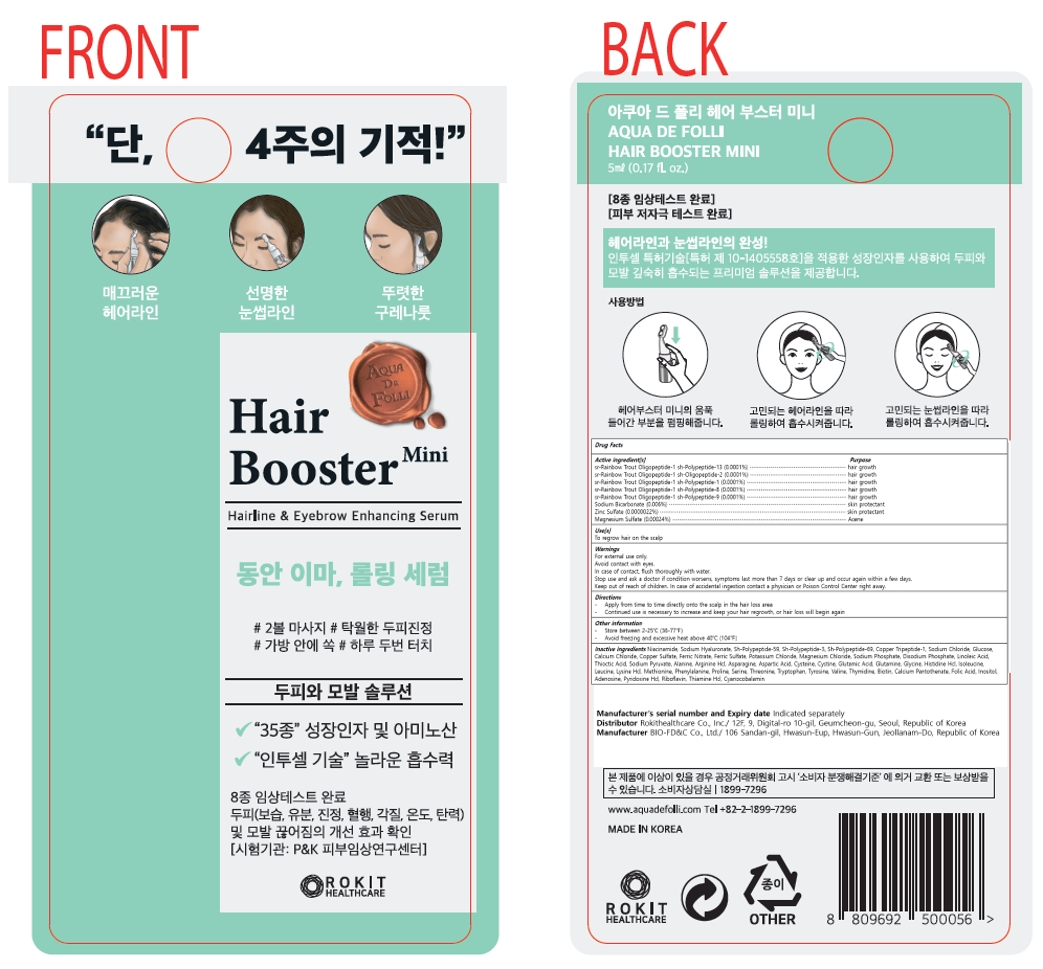
Active ingredient[s]
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-13 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Oligopeptide-2 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-1 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-8 (0.0001%)
sr-Rainbow Trout Oligopeptide-1 sh-Polypeptide-9 (0.0001%)
Sodium Bicarbonate (0.006%)
Zinc Sulfate (0.0000022%)
Magnesium Sulfate (0.00024%)
Warnings
For external use only.
Avoid contact with eyes.
In case of contact, flush thoroughly with water.
Warnings
Stop use and ask a doctor if condition worsens, symptoms last more than 7 days or clear up and occur again within a few days.
Warnings
Keep out of reach of children. In case of accidental ingestion contact a physician or Poison Control Center right away.
Directions
- Apply from time to time directly onto the scalp in the hair loss area
- Continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again
Other information
- Store between 2-25℃ (36-77℉)
- Avoid freezing and excessive heat above 40℃ (104℉)
Inactive ingredients
Niacinamide, Sodium Hyaluronate, Sh-Polypeptide-59, Sh-Polypeptide-3, Sh-Polypeptide-69, Copper Tripeptide-1, Sodium Chloride, Glucose, Calcium Chloride, Copper Sulfate, Ferric Nitrate, Ferric Sulfate, Potassium Chloride, Magnesium Chloride, Sodium Phosphate, Disodium Phosphate, Linoleic Acid, Thioctic Acid, Sodium Pyruvate, Alanine, Arginine Hcl, Asparagine, Aspartic Acid, Cysteine, Cystine, Glutamic Acid, Glutamine, Glycine, Histidine Hcl, Isoleucine, Leucine, Lysine Hcl, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, Valine, Thymidine, Biotin, Calcium Pantothenate, Folic Acid, Inositol, Adenosine, Pyridoxine Hcl, Riboflavin, Thiamine Hcl, Cyanocobalamin, Coceth-7, PEG-40 Hydrogenated Castor Oil, PPG-1-PEG-9 Lauryl Glycol Ether