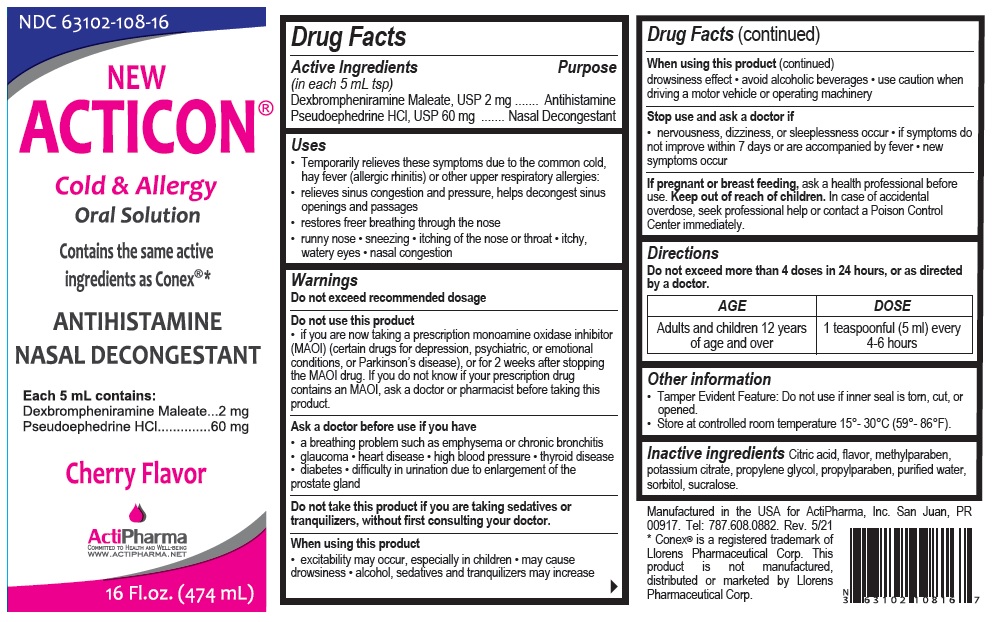
Label: ACTICON- dexbrompheniramine maleate, pseudoephedrine hydrochloride solution
- NDC Code(s): 63102-108-16
- Packager: ACTIPHARMA, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients (in each 5 mL tsp)
- Purpose
-
Uses
• Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
• relieves sinus congestion and pressure, helps decongest sinus openings and passages
• restores freer breathing through the nose
• runny nose • sneezing • itching of the nose or throat • itchy, watery eyes • nasal congestion -
Warnings
Do not exceed recommended dosage
Do not use this product
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
• a breathing problem such as emphysema or chronic bronchitis • glaucoma • heart disease • high blood pressure • thyroid disease • diabetes • difficulty in urination due to enlargement of the prostate gland
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
• excitability may occur, especially in children • may cause drowsiness • alcohol, sedatives and tranquilizers may increase drowsiness effect • avoid alcoholic beverages • use caution when driving a motor vehicle or operating machineryStop use and ask a doctor if
• nervousness, dizziness, or sleeplessness occur • if symptoms do not improve within 7 days or are accompanied by fever • new symptoms occurIf pregnant or breast feeding, ask a health professional before use.
- Directions
- Other information
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
Contains the same active ingredients as Conex®*
Cherry Flavor
ActiPharma
COMMITTED TO HEALTH AND WELL-BEING
WWW.ACTIPHARMA.NETManufactured in the USA for ActiPharma, Inc. San Juan, PR 00917. Tel: 787.608.0882. Rev. 5/21
* Conex® is a registered trademark of Llorens Pharmaceutical Corp. This product is not manufactured, distributed or marketed by Llorens Pharmaceutical Corp. - Packaging
-
INGREDIENTS AND APPEARANCE
ACTICON
dexbrompheniramine maleate, pseudoephedrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63102-108 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXBROMPHENIRAMINE MALEATE (UNII: BPA9UT29BS) (DEXBROMPHENIRAMINE - UNII:75T64B71RP) DEXBROMPHENIRAMINE MALEATE 2 mg in 5 mL PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63102-108-16 474 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/15/2022 Labeler - ACTIPHARMA, INC (079340948)