Label: FOAMYIQ HEALTHCARE PERSONNEL HANDWASH- chloroxyenol soap
- NDC Code(s): 64009-203-06
- Packager: Spartan Chemical Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions?
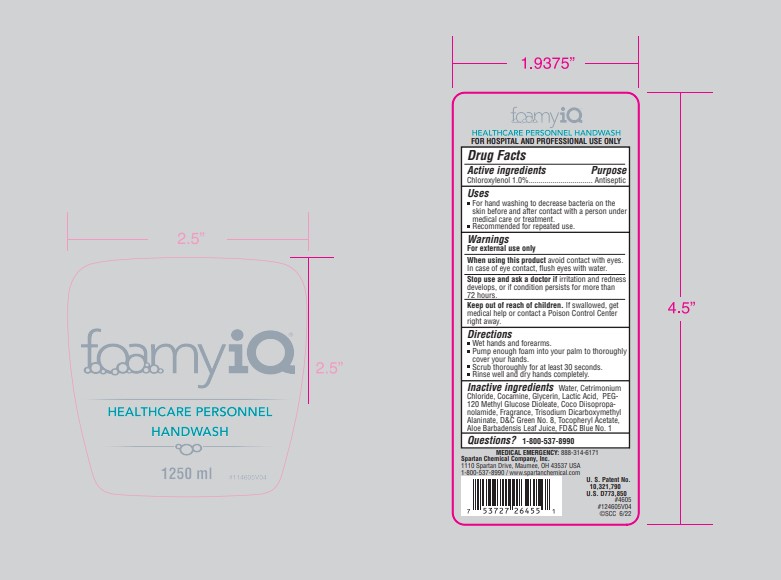
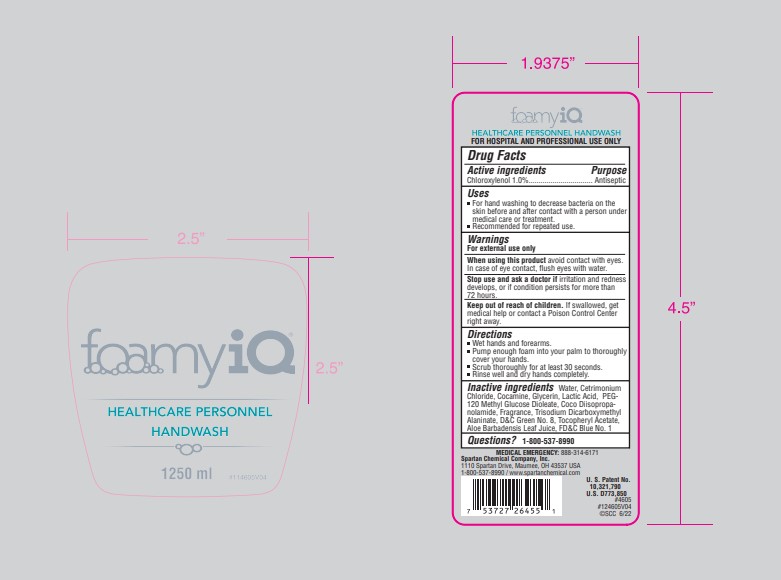
- Principal Display Panel - 1.25 L Container Label
-
INGREDIENTS AND APPEARANCE
FOAMYIQ HEALTHCARE PERSONNEL HANDWASH
chloroxyenol soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64009-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 10.11 g in 1 L Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) COCAMINE (UNII: 6724Q5747W) GLYCERIN (UNII: PDC6A3C0OX) LACTIC ACID, L- (UNII: F9S9FFU82N) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) COCO DIISOPROPANOLAMIDE (UNII: S485AM948Q) TRISODIUM DICARBOXYMETHYL ALANINATE, L- (UNII: L754QR50CZ) D&C GREEN NO. 8 (UNII: I2W85YOX9L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64009-203-06 1.25 L in 1 CONTAINER; Type 0: Not a Combination Product 12/09/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/09/2019 Labeler - Spartan Chemical Company (005036728) Establishment Name Address ID/FEI Business Operations Spartan Chemical Company 005036728 manufacture(64009-203)

 foamy
foamy