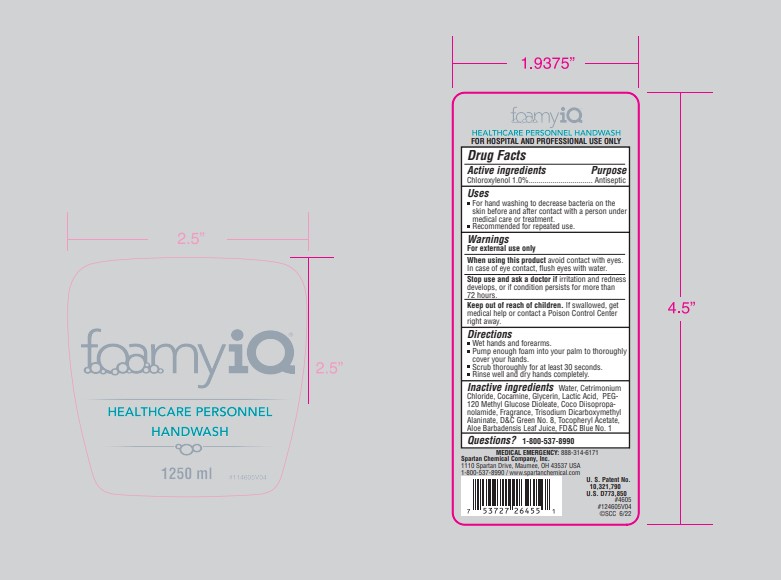
Active Ingredients
Chloroxylenol 1.0%
Uses
- Healthcare personnel hand wash for hand washing to decrease bacteria on the skin before and after contact with a person under medical care or treatment.
- Recommended for repeated use.
Warnings
When using this product avoid contact with eyes. In case of eye contact, flush eyes with water.
Stop use and ask a doctor if irritation and redness develops, or if condition persists for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands and forearms.
- Pump enough foam into your palm to thoroughly cover your hands.
- Scrub thoroughly for at least 30 seconds.
- Rinse well and dry hands completely.
Inactive Ingredients
Water, Cetrimonium Chloride, Cocamine, Glycerin, Lactic Acid, PEG-120 Methyl Glucose Dioleate, Coco Diisopropa- nolamide, Fragrance, Trisodium Dicarboxymethyl Alaninate, D&C Green No. 8, Tocopheryl Acetate, Aloe Barbadensis Leaf Juice, FD&C Blue No. 1
Questions?
1-800-537-8990
Principal Display Panel - 1.25 L Container Label
 foamy
iQ™
foamy
iQ™
HEALTHCARE PERSONNEL
HANDWASH
#114605V04
 foamy
foamy