Label: BONINE- meclizine hydrochloride tablet, chewable
-
NDC Code(s):
65197-275-02,
65197-275-08,
65197-275-12,
65197-275-16, view more65197-296-08, 65197-296-12, 65197-296-16, 65197-296-24, 65197-296-32
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
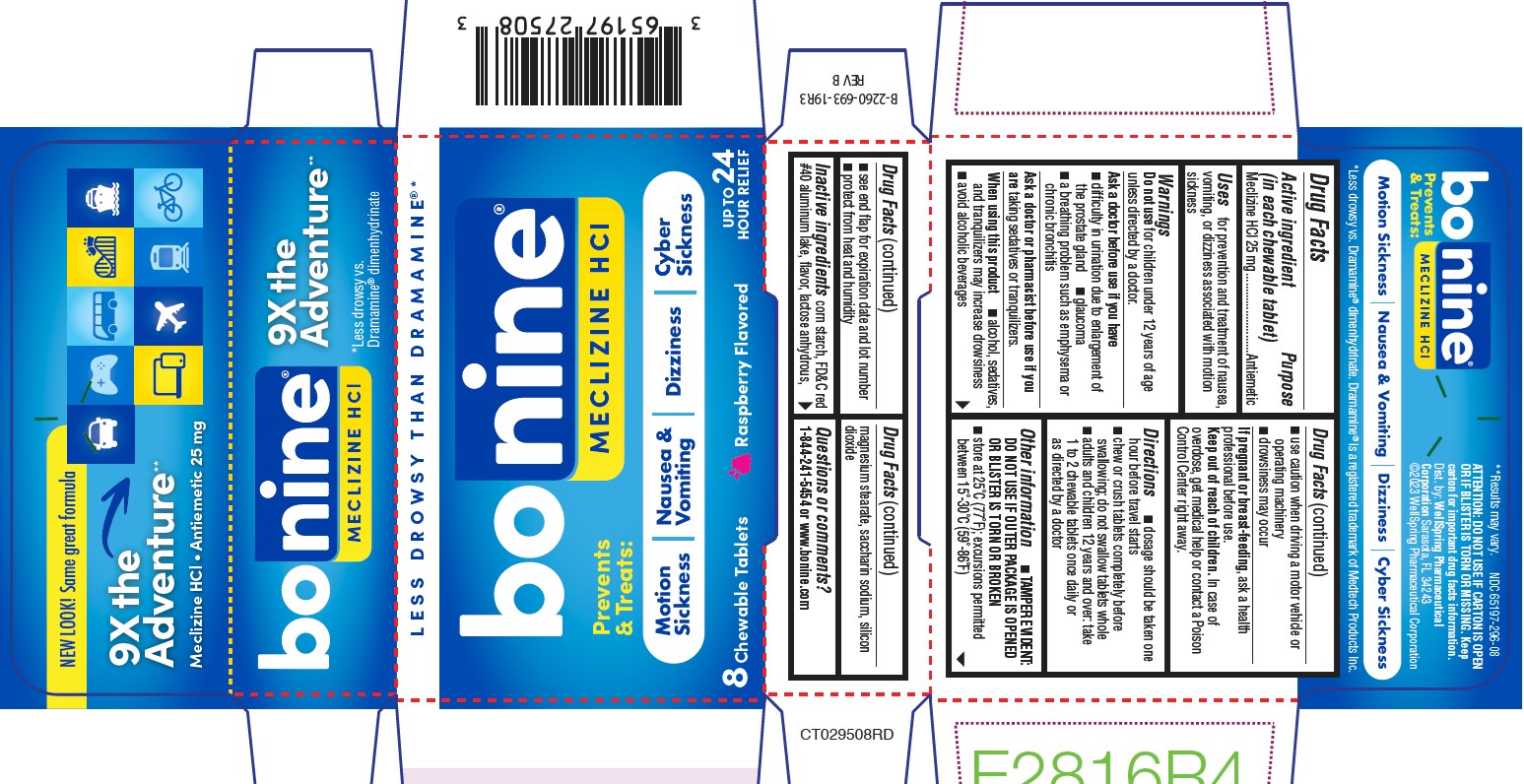
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not take this product, unless directed by a doctor, if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Do not take this product if you are
taking sedatives or tranquilizers, without first consulting your doctor.
- Directions (65197-275)
- Directions (65197-296)
- Other information
- Inactive ingredients (65197-275)
- Inactive Ingredients (65197-296)
- Questions?
- TAMPER EVIDENT 65197-275
- TAMPER EVIDENT 65197-296
- Dist. by:
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 65197-275
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 65197-296
-
INGREDIENTS AND APPEARANCE
BONINE
meclizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-275 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (UNII: 2S7830E561) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SACCHARIN SODIUM (UNII: SB8ZUX40TY) STEARIC ACID (UNII: 4ELV7Z65AP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) RASPBERRY (UNII: 4N14V5R27W) VANILLA (UNII: Q74T35078H) Product Characteristics Color pink (light pink) Score 2 pieces Shape ROUND Size 9mm Flavor RASPBERRY, VANILLA Imprint Code Bonine;201 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-275-08 1 in 1 BOX 12/15/2014 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:65197-275-12 1 in 1 BOX 12/15/2014 2 12 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:65197-275-16 2 in 1 BOX 12/15/2014 3 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:65197-275-02 2 in 1 POUCH; Type 0: Not a Combination Product 12/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 12/15/2014 BONINE
meclizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-296 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) FD&C RED NO. 40 (UNII: WZB9127XOA) RASPBERRY (UNII: 4N14V5R27W) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color pink Score 2 pieces Shape ROUND Size 9mm Flavor RASPBERRY Imprint Code Bonine;201 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-296-08 1 in 1 BOX 02/15/2023 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:65197-296-12 1 in 1 BOX 02/15/2023 2 12 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:65197-296-16 2 in 1 BOX 02/15/2023 3 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:65197-296-24 3 in 1 CARTON 05/01/2023 4 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:65197-296-32 4 in 1 CARTON 06/01/2023 5 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 02/15/2023 Labeler - WellSpring Pharmaceutical Corporation (110999054)