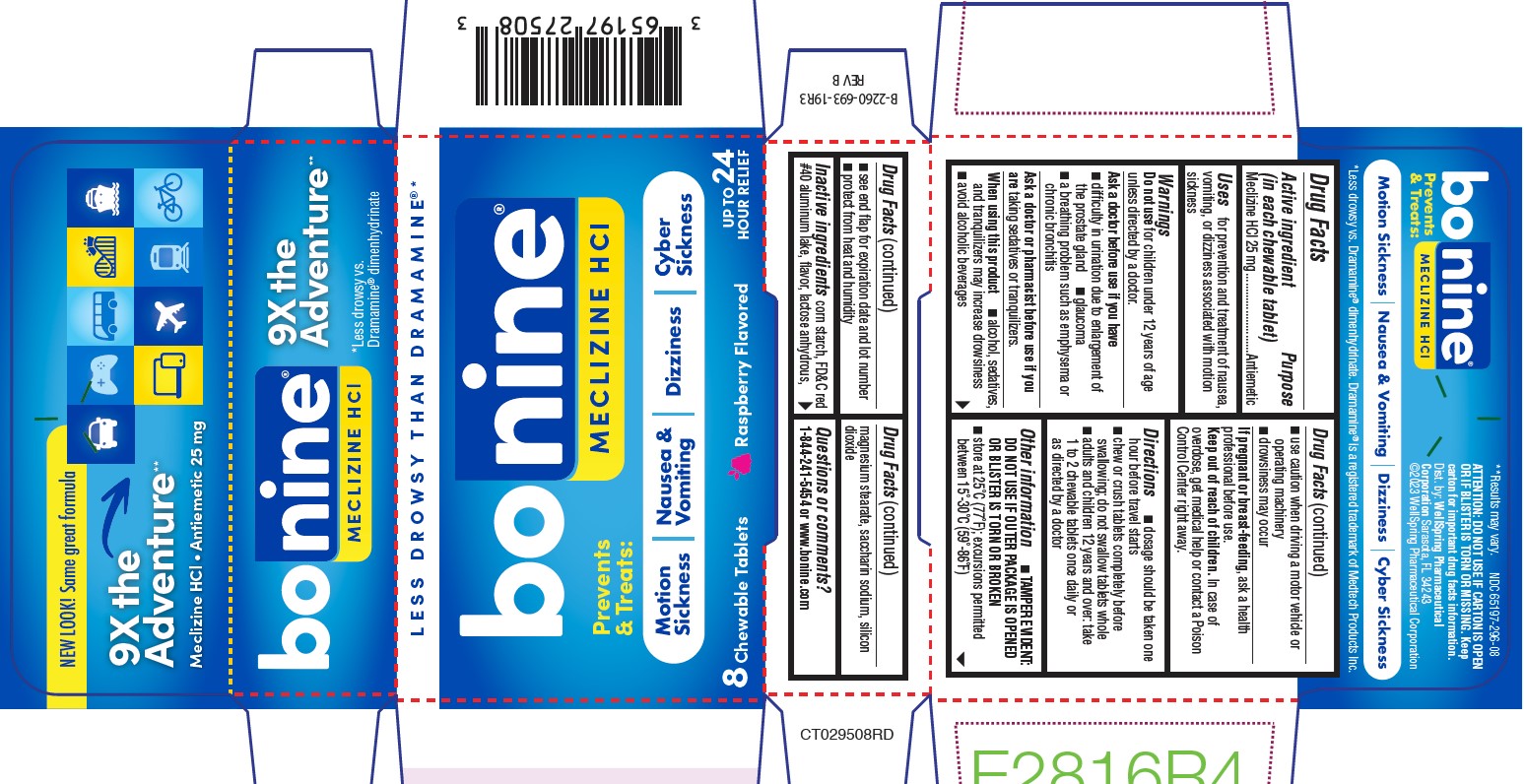
Warnings
Do not take this product, unless directed by a doctor, if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Do not take this product if you are
taking sedatives or tranquilizers, without first consulting your doctor.
Directions (65197-275)
- dosage should be taken one hour before travel starts
- adults and children 12 years of age and over: take 1 to 2 tablets once daily or as directed by a doctor
Directions (65197-296)
- dosage should be taken one hour before travel starts
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- adults and children 12 years and over: take 1 to 2 chewable tablets once daily or as directed by a doctor
Other information
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
Inactive ingredients (65197-275)
croscarmellose sodium, crospovidone, FD&C red #40 lake, lactose, magnesium stearate, raspberry flavor, silica, sodium saccharin, stearic acid, vanilla flavor.
Inactive Ingredients (65197-296)
corn starch, FD&C red #40 aluminum lake, flavor, lactose anhydrous, magnesium stearate, saccharin sodium, silicon dioxide
TAMPER EVIDENT 65197-275
TAMPER EVIDENT: DO NOT USE IF TAMPER EVIDENCE TAPE OVER CAP IS BROKEN OR MISSING.
TAMPER EVIDENT 65197-296
ATTENTION: DO NOT USE IF CARTON IS OPEN OR IF BLISTER IS TORN OR MISSING.
Keep Carton for important drug facts information.
Dist. by:
WellSpring Pharmaceutical
Corporation Sarasota, FL 34243
©2023 WellSpring Pharmaceutical Corporation
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 65197-275
UP TO 24 HOUR PROTECTION
BONINE®
MECLIZINE HYDROCHLORIDE • ANTIEMETIC
Nausea - Dizziness - Vomiting
*Less drowsy than Dramamine

Bonine 12 ct
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 65197-296
NEW LOOK! Same great formula
9X the Adventure**
**Results may vary.
Meclizine HCL • Antiemetic 25mg
*Less drowsy than Dramamine ®
BONINE®
MECLIZINE HCL
Prevents & Treats: Motion Sickness / Nausea & Vomiting / Dizziness / Cyber Sickness
Up to 24 Hours Relief
Bonine 8ct Blue new Design