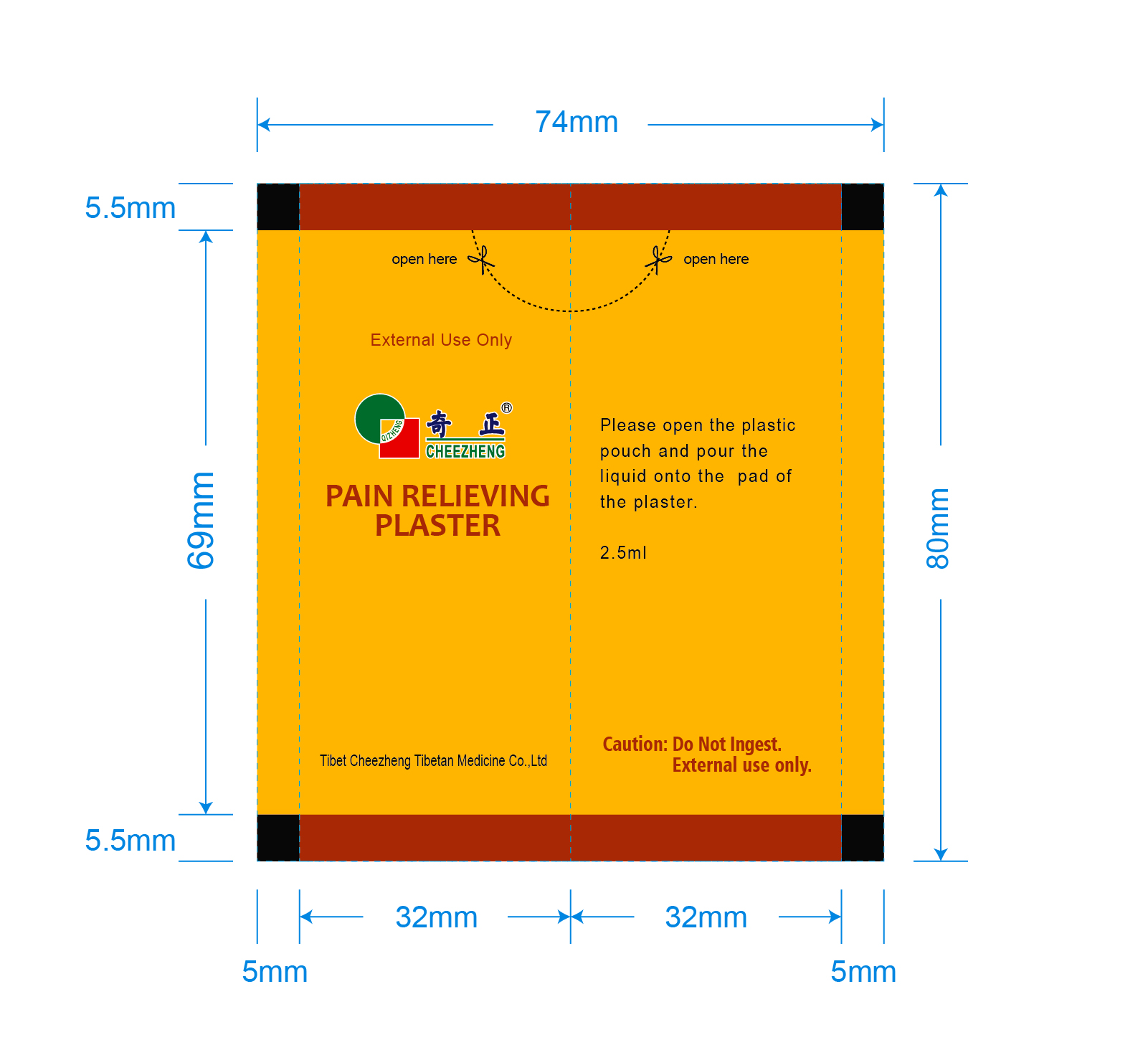
Label: CHEEZHENG PAIN RELIEVEING PLASTER- camphor plaster
- NDC Code(s): 66506-183-01, 66506-183-02
- Packager: Tibet Cheezheng Tibetan Medicine Co. Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 20, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- ASK DOCTOR
- Keep out of reach of children
- If pregnant
-
Directions
■Adults and children 12 years of age and older: remove protective film from the plaster, pour the liquid onto the pad of plaster and apply it to the painful area or acupoint as directed (see figure)
■Use 1 to 2 plasters every 24 hours
■Apply the plaster to the affected area for 4 to 8 hours
■Do not apply to area with excessive hair. Adhesive plaster may hurt skin upon removal.
■Children under 12 years of age: do not use or consult a doctor. - Other information
- Inactive ingredients
- Questions?
- Purpose
- Do not use
- When using this product
- Stop use and ask a doctor if
- Warnings
- Uses
- CHEEZHENG PRP OUTER BOX
-
INGREDIENTS AND APPEARANCE
CHEEZHENG PAIN RELIEVEING PLASTER
camphor plasterProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66506-183 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 1 g in 100 g Inactive Ingredients Ingredient Name Strength ZANTHOXYLUM BUNGEANUM FRUIT RIND (UNII: K447936H97) CURCUMA LONGA WHOLE (UNII: W5488JUO8U) CARTHAMUS TINCTORIUS FLOWER BUD (UNII: B86IS274O0) PHLOMOIDES ROTATA WHOLE (UNII: 7CEU5N8573) MYRICARIA PANICULATA STEM (UNII: 4C36LPC9I2) OXYTROPIS FALCATA WHOLE (UNII: 7276D7W5GN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66506-183-01 5 in 1 PACKAGE 11/29/2019 1 NDC:66506-183-02 1.2 g in 1 PACKAGE; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/29/2019 Labeler - Tibet Cheezheng Tibetan Medicine Co. Ltd. (529074851) Establishment Name Address ID/FEI Business Operations Cheezheng Tibetan Medicine Co. Ltd. 529074851 manufacture(66506-183)