CHEEZHENG PAIN RELIEVEING PLASTER- camphor plaster
Tibet Cheezheng Tibetan Medicine Co. Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Camphor 1%.................……….…….External analgesic
Do not use otherwise than as directed
Keep out of reach of children
Keep out of reach of children to avoid accidental poisoning
If pregnant
If pregnant, ask a health professional before use
Directions
■Adults and children 12 years of age and older: remove protective film from the plaster, pour the liquid onto the pad of plaster and apply it to the painful area or acupoint as directed (see figure)
■Use 1 to 2 plasters every 24 hours
■Apply the plaster to the affected area for 4 to 8 hours
■Do not apply to area with excessive hair. Adhesive plaster may hurt skin upon removal.
■Children under 12 years of age: do not use or consult a doctor.
Other information
Store at room temperature, 10 ℃ to 30℃ (50℉ to 86℉)
Inactive ingredients
Zanthoxylum bungeanum Maxim, Lamiophlomis rotata (Benth.) Kudo, Curcuma longa L, Carthamus tinctorius L, Myricaria germanica (L.) Desv, Oxytropis falcata Bunge
Questions?
+1-888-915-6789
Purpose
External analgesic
Do not use
- on wounds
- irritated or damaged skin
- sensitive skin
When using this product
- avoid contact with the eyes
- do not use more than 2 plasters a day
- do not use 1 hour before or after bathing
Stop use and ask a doctor if
- Condition worsens
- Symptoms persist for more than 7 days
- Symptoms clear up and occur again within a few days
- Eexcessive irritation of the skin develops
- Redness is present
Warnings
For external use only
Uses
For the temporary relief of minor pain.
CHEEZHENG PRP OUTER BOX

InnerPackage
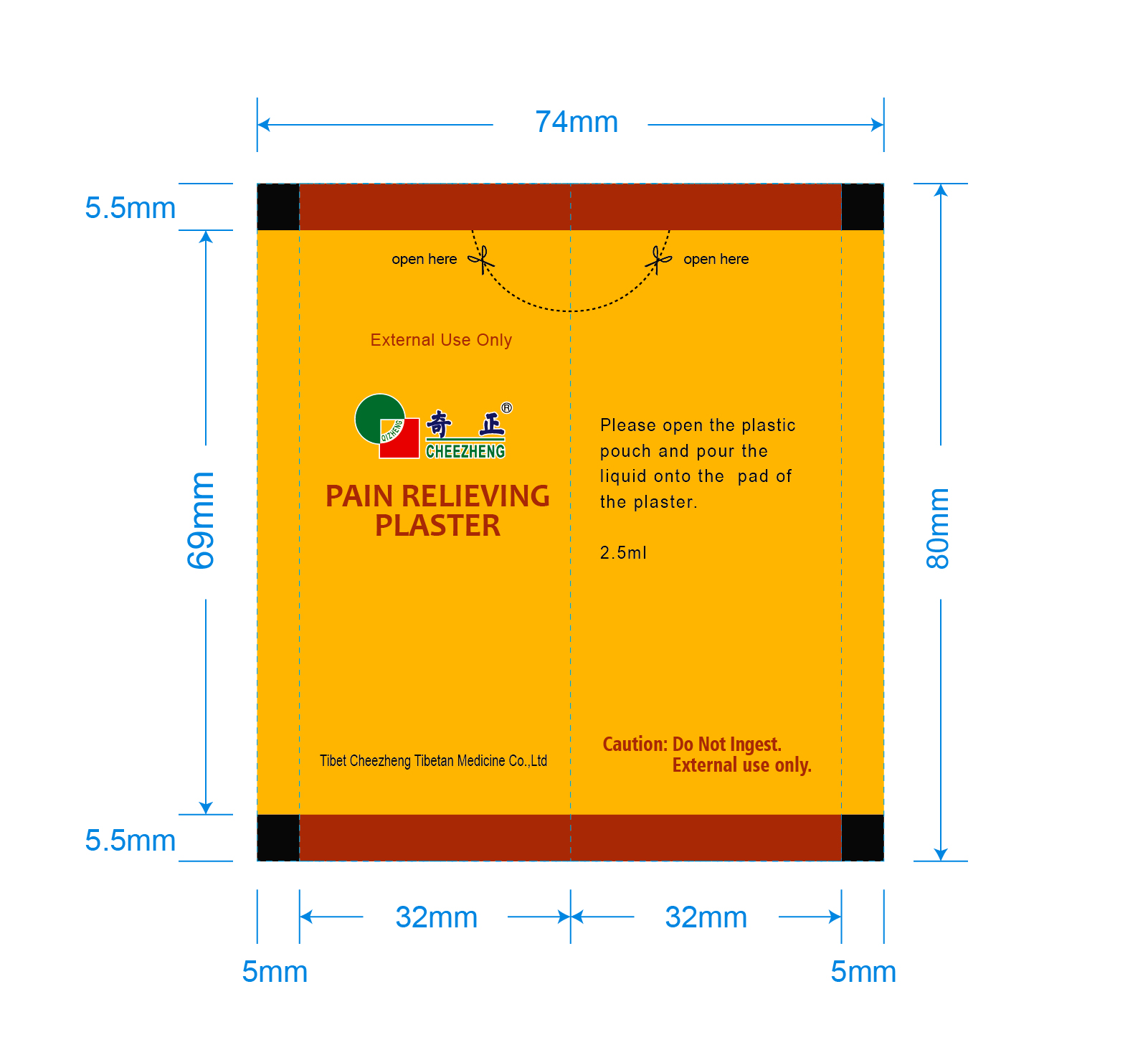
CHEEZHENG PRP INNER BAG

Tibet Cheezheng Tibetan Medicine Co. Ltd.


