Label: SHEFFIELD ADVANCED SCAR GEL- advanced scar gel gel
- NDC Code(s): 11527-725-07
- Packager: Sheffield Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 30, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredient
- Principal Display Panel –Tube
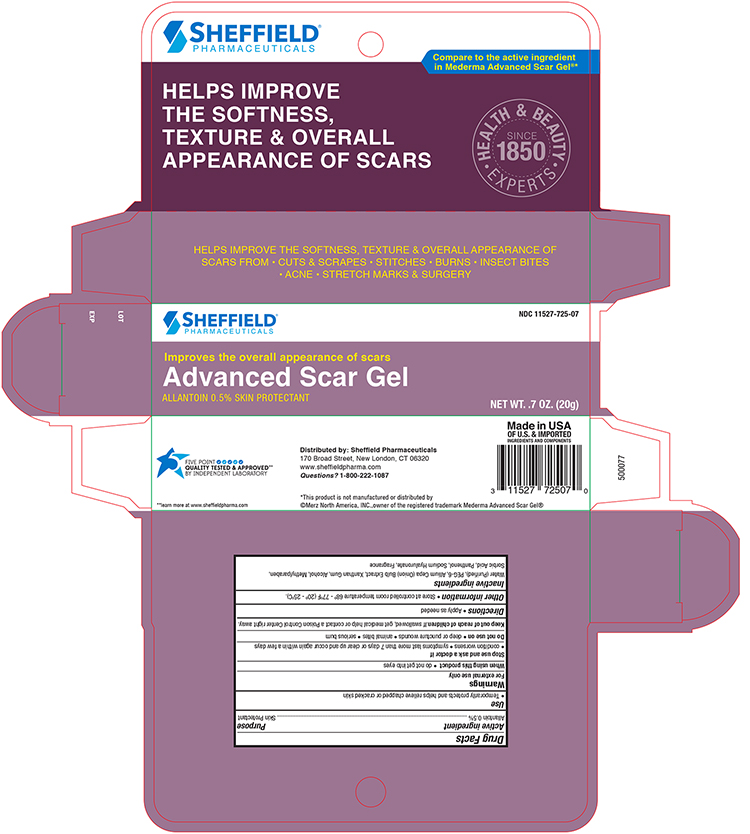
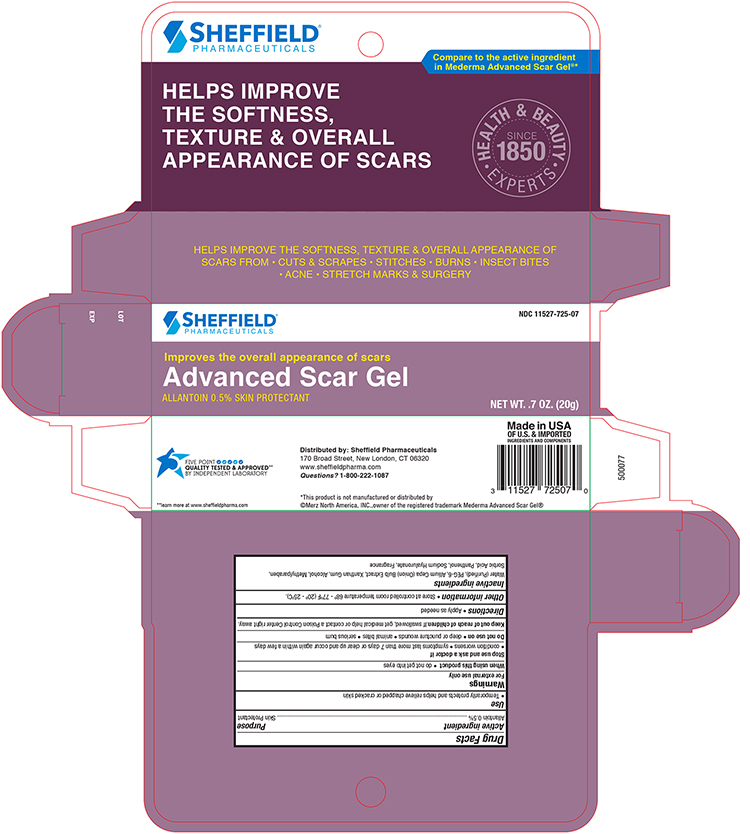
- Principal Display Panel – Carton
-
INGREDIENTS AND APPEARANCE
SHEFFIELD ADVANCED SCAR GEL
advanced scar gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11527-725 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLOXAMER 407 (UNII: TUF2IVW3M2) ONION (UNII: 492225Q21H) XANTHAN GUM (UNII: TTV12P4NEE) ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) Sorbic Acid (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11527-725-07 1 in 1 CARTON 10/14/2021 1 20 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/14/2021 Labeler - Sheffield Pharmaceuticals LLC (151177797) Registrant - Sheffield Pharmaceuticals LLC (151177797) Establishment Name Address ID/FEI Business Operations Sheffield Pharmaceuticals LLC 151177797 MANUFACTURE(11527-725)