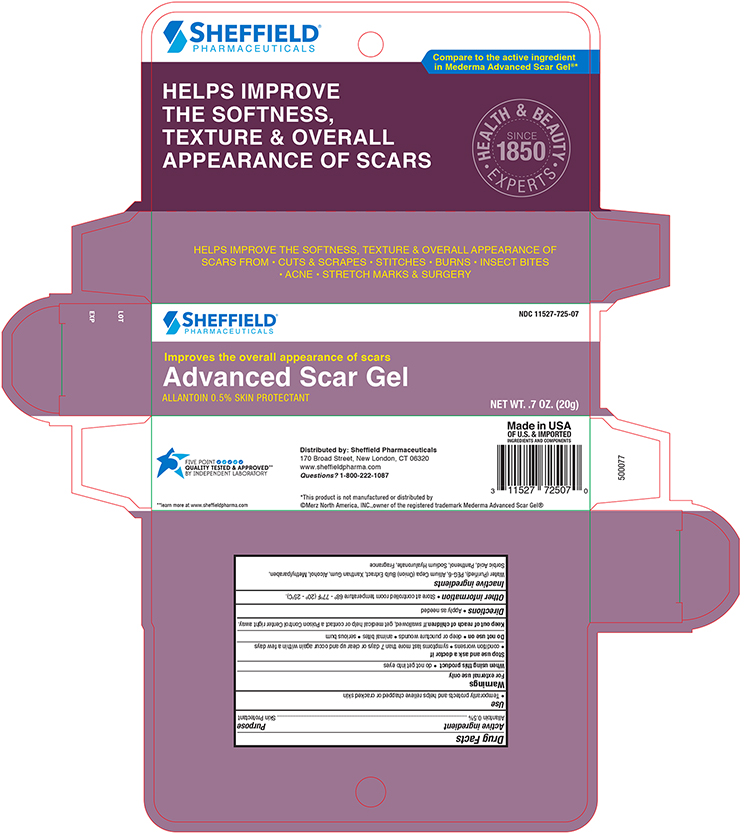
Active ingredients
Allantion 0.5%
Uses
Temporarily protects and helps chapped or cracked skin
Warnings
For external use only
Stop Use and Ask a Doctor if
- condition worsens
- symptoms last for more than 7 days or clears up and occur again within a few days
Do Not Use on
- deep or puncture wounds
- animal bites
- serious burns
Keep out of the reach of children. If swallowed get medical help or contact a Poison Control Center immediately.
Directions
Apply as needed
Other information
- Store at room temperature
Inactive ingredient
Water (Purified), PEG 300, Allium Cepa (Onion) Bulb Extract, Xanthan Gum, Alcohol, Methylparaben, Sorbic Acid, Panthenol, Sodium Hyaluronate, Fragrance
Principal Display Panel –Tube
Sheffield NDC 11527-72-07
Advanced Scar Gel
0.5% Allantoin
NET WT. 0.70 oz (20g)

Principal Display Panel – Carton
Sheffield NDC 11527-725-07
Advance Scar Gel
Skin Protectant
NET WT. 0.70 oz (20g)