Label: DIABECLINE- tetracycline hydrochloride ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 24471-200-10, 24471-200-30 - Packager: THRU PHARMA, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 23, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
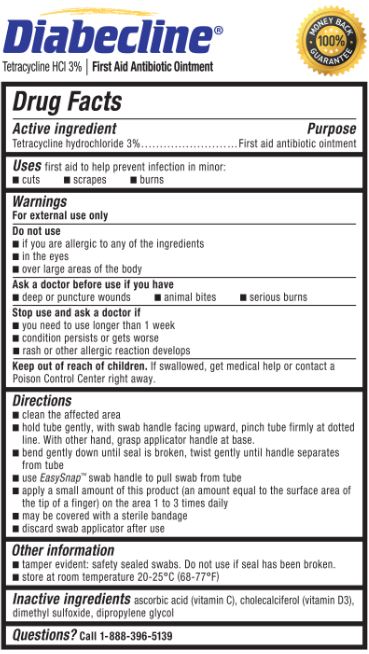
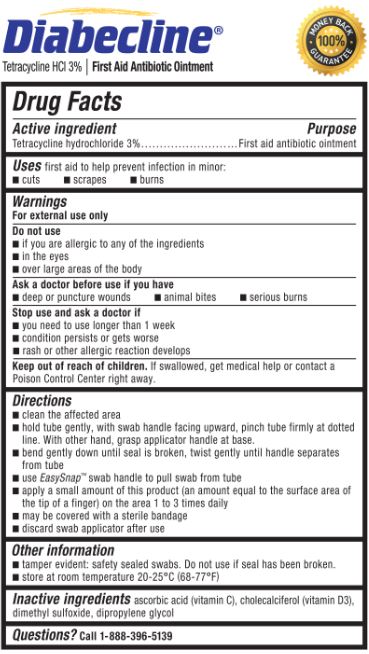
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- clean the affected area
-
hold the tube gently, with swab handle facing upward, pinch tube firmly at dotted line. With other hand, grasp applicator handle at base.
-
bend gently down until seal is broken, twist gently until handle separates from tube
-
use EasySnap™ handle to pull swab from tube
-
apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
-
may be covered with a sterile bandage
-
discard swab applicator after use
- Other information
- Inactive ingredients
- Questions?
-
Principal display panel
NEW! NDC 24471-200-10
Diabecline®
Tetracycline HCl 3% | First Aid Antibiotic Ointment
The Power to Protect.
Maximum Strength
First Aid Antibiotic Ointment
24 Hour Infection Protection
10 EasySnap™ Swabs
0.2 ml eachMaximum strength hypoallergenic topical first aid antibiotic
Broad-spectrum dual-action antibiotic kills bacteria, creates a zone of protection
Convenient EasySnap™ swab application for extra reach and precise delivery
Questions? Visit diabecline.com or call 1-888-396-5139
100% MONEY BACK GUARANTEE
PharmaCline
Diabecline®
Tetracycline HCl 3% | First Aid Antibiotic Ointment
100% MONEY BACK GUARANTEE
Diabecline®
Tetracycline HCl 3% | First Aid Antibiotic Ointment
with Vitamin D
1 EasySnap™ Swab 0.2 ml
See package insert for full product Uses, Directions and Warnings.
Store at 20-25°C (68-77°F)
Tamper evident: do not use if cap on swab is broken or missing
Dist. by: pharmaCline, Sioux Falls, SD 57107
©pharmaCline 2012 1-888-396-5139
PINCH HERE, SNAP & PULL OUT
-
INGREDIENTS AND APPEARANCE
DIABECLINE
tetracycline hydrochloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24471-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tetracycline Hydrochloride (UNII: P6R62377KV) (Tetracycline - UNII:F8VB5M810T) Tetracycline Hydrochloride 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength Ascorbic Acid (UNII: PQ6CK8PD0R) Cholecalciferol (UNII: 1C6V77QF41) Dimethyl Sulfoxide (UNII: YOW8V9698H) Dipropylene Glycol (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24471-200-10 10 in 1 BOX 1 .2 mL in 1 APPLICATOR 2 NDC:24471-200-30 30 in 1 BOX 2 .2 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 05/23/2013 Labeler - THRU PHARMA, LLC (968309232) Establishment Name Address ID/FEI Business Operations Great Midwest Packaging, LLC 784514502 REPACK(24471-200) Establishment Name Address ID/FEI Business Operations Continental Manufacturing Chemist, Inc. 005278007 MANUFACTURE(24471-200)