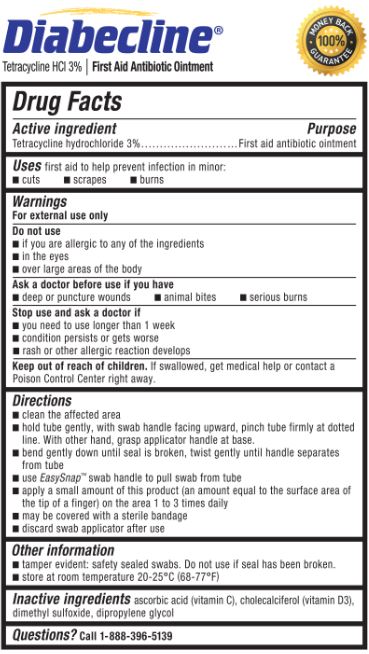
Stop use and ask a doctor if
- you need to use longer than 1 week
- condition persists or gets worse
- rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area
-
hold the tube gently, with swab handle facing upward, pinch tube firmly at dotted line. With other hand, grasp applicator handle at base.
-
bend gently down until seal is broken, twist gently until handle separates from tube
-
use EasySnap™ handle to pull swab from tube
-
apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
-
may be covered with a sterile bandage
-
discard swab applicator after use
Other information
- tamper evident: safety sealed swabs. Do not use if seal has been broken.
- store at room temperature 20-25°C (68-77°F)
Inactive ingredients
ascorbic acid (vitamin C), cholecalciferol (vitamin D3), dimethyl sulfoxide, dipropylene glycol
Principal display panel
NEW! NDC 24471-200-10
Diabecline®
Tetracycline HCl 3% | First Aid Antibiotic Ointment
The Power to Protect.
Maximum Strength
First Aid Antibiotic Ointment
24 Hour Infection Protection
10 EasySnap™ Swabs
0.2 ml each
Maximum strength hypoallergenic topical first aid antibiotic
Broad-spectrum dual-action antibiotic kills bacteria, creates a zone of protection
Convenient EasySnap™ swab application for extra reach and precise delivery
Questions? Visit diabecline.com or call 1-888-396-5139
100% MONEY BACK GUARANTEE
PharmaCline

Diabecline®
Tetracycline HCl 3% | First Aid Antibiotic Ointment
100% MONEY BACK GUARANTEE
Diabecline®
Tetracycline HCl 3% | First Aid Antibiotic Ointment
with Vitamin D
1 EasySnap™ Swab 0.2 ml
See package insert for full product Uses, Directions and Warnings.
Store at 20-25°C (68-77°F)
Tamper evident: do not use if cap on swab is broken or missing
Dist. by: pharmaCline, Sioux Falls, SD 57107
©pharmaCline 2012 1-888-396-5139
PINCH HERE, SNAP & PULL OUT
