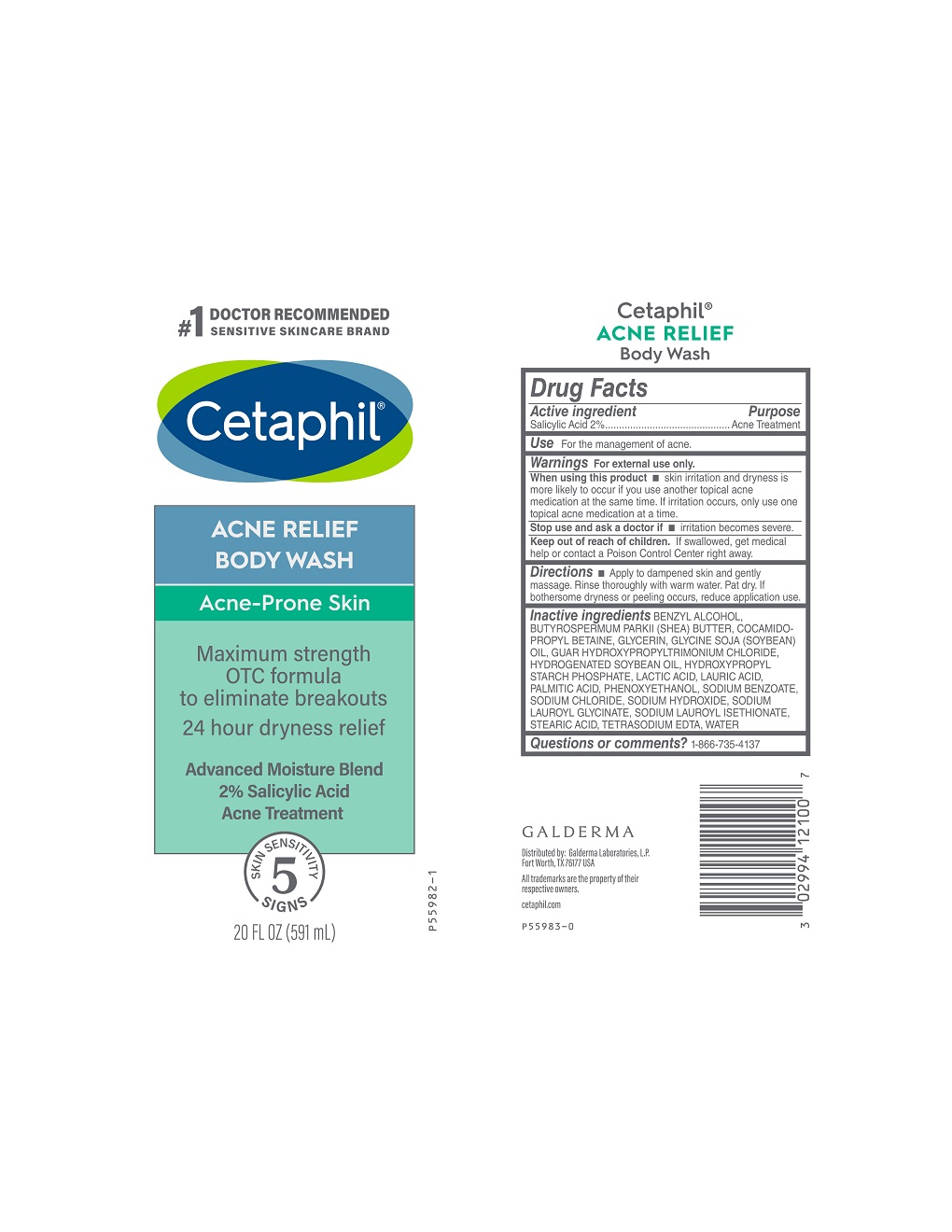
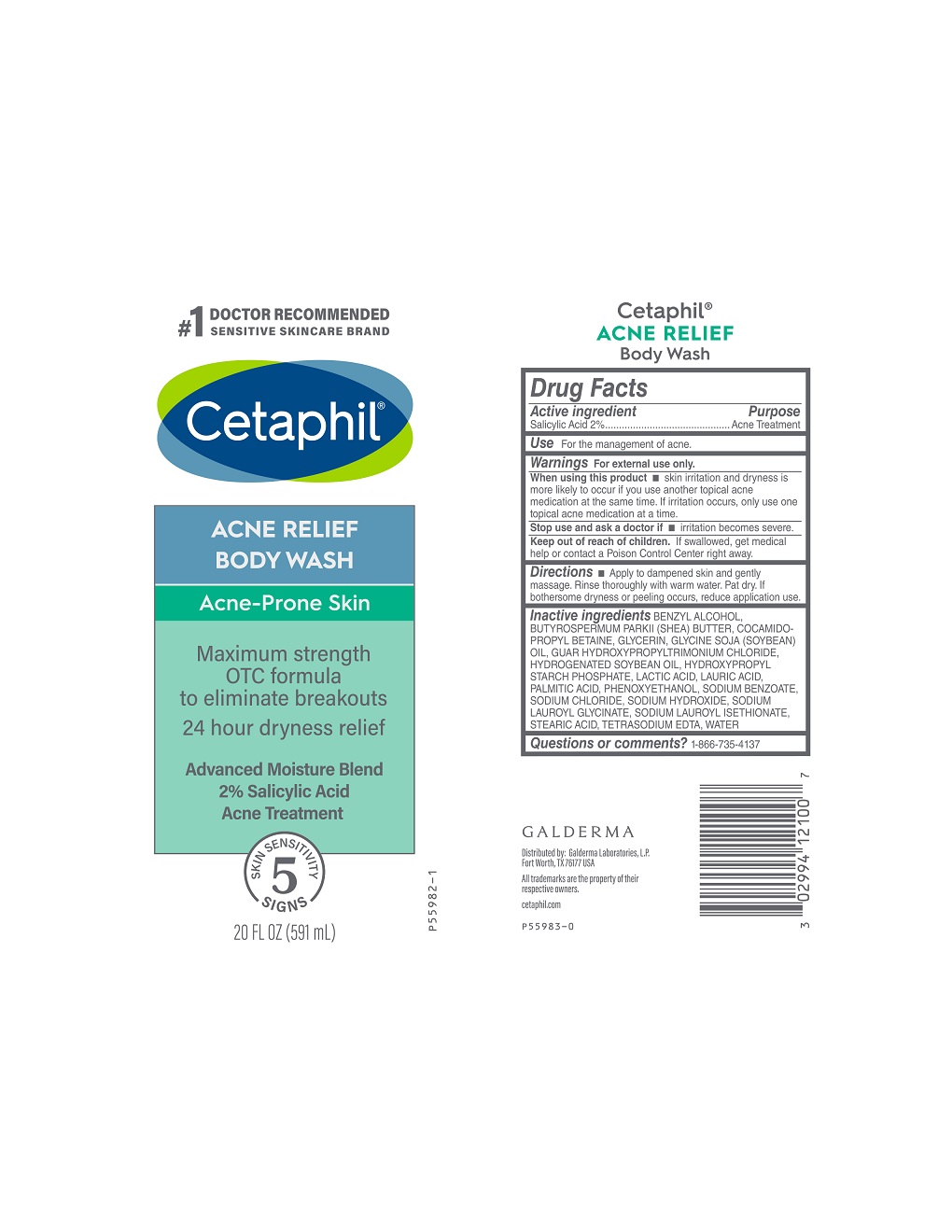
Label: CETAPHIL ACNE RELIEF BODY WASH- salicylic acid gel
- NDC Code(s): 0299-4121-00
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
-
Inactive Ingredients
BENZYL ALCOHOL, BUTYROSPERMUM PARKII (SHEA) BUTTER, COCAMIDOPROPYL BETAINE, GLYCERIN, GLYCINE SOJA (SOYBEAN) OIL, GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE, HYDROGENATED SOYBEAN OIL, HYDROXYPROPYL STARCH PHOSPHATE, LACTIC ACID, LAURIC ACID, PALMITIC ACID, PHENOXYETHANOL, SODIUM BENZOATE, SODIUM CHLORIDE, SODIUM HYDROXIDE, SODIUM LAUROYL GLYCINATE, SODIUM LAUROYL ISETHIONATE, STEARIC ACID, TETRASODIUM EDTA, WATER
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 20 FL OZ bottle
-
INGREDIENTS AND APPEARANCE
CETAPHIL ACNE RELIEF BODY WASH
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzyl Alcohol (UNII: LKG8494WBH) Shea Butter (UNII: K49155WL9Y) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Glycerin (UNII: PDC6A3C0OX) Soybean Oil (UNII: 241ATL177A) Guar Hydroxypropyltrimonium Chloride (1.7 Substituents Per Saccharide) (UNII: B16G315W7A) Hydrogenated Soybean Oil (UNII: A2M91M918C) Lactic Acid, Unspecified Form (UNII: 33X04XA5AT) Lauric Acid (UNII: 1160N9NU9U) Palmitic Acid (UNII: 2V16EO95H1) Phenoxyethanol (UNII: HIE492ZZ3T) Sodium Benzoate (UNII: OJ245FE5EU) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Sodium Lauroyl Glycinate (UNII: L54QIO80PN) Sodium Lauroyl Isethionate (UNII: M590021Z02) Stearic Acid (UNII: 4ELV7Z65AP) Edetate Sodium (UNII: MP1J8420LU) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4121-00 591 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/01/2022 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Fruit of The Earth, Inc 080086802 manufacture(0299-4121)