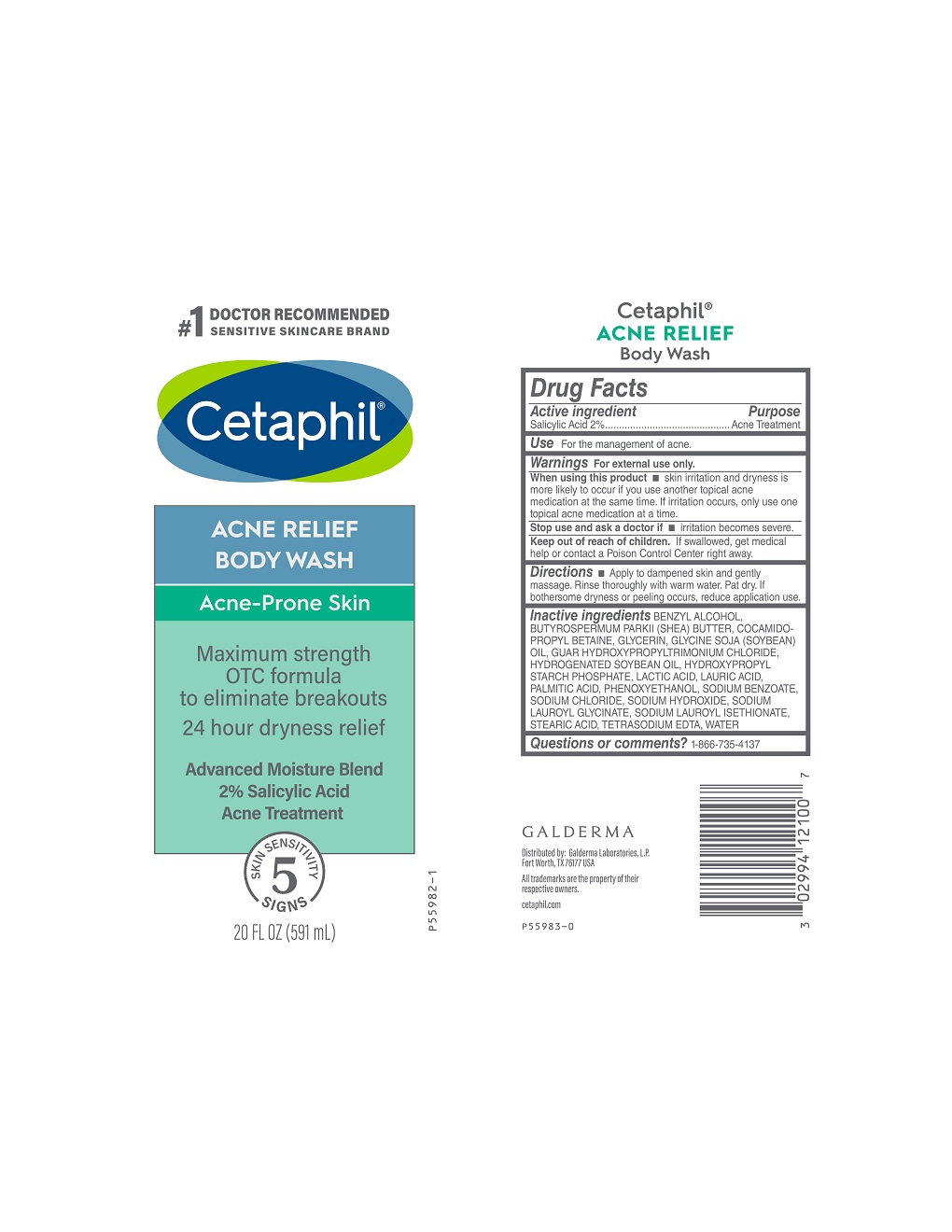
When using this product
• skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply to dampened skin and gently massage. Rinse thoroughly with warm water. Pat dry. If bothersome dryness or peeling occurs, reduce application use.
Inactive Ingredients
BENZYL ALCOHOL, BUTYROSPERMUM PARKII (SHEA) BUTTER, COCAMIDOPROPYL BETAINE, GLYCERIN, GLYCINE SOJA (SOYBEAN) OIL, GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE, HYDROGENATED SOYBEAN OIL, HYDROXYPROPYL STARCH PHOSPHATE, LACTIC ACID, LAURIC ACID, PALMITIC ACID, PHENOXYETHANOL, SODIUM BENZOATE, SODIUM CHLORIDE, SODIUM HYDROXIDE, SODIUM LAUROYL GLYCINATE, SODIUM LAUROYL ISETHIONATE, STEARIC ACID, TETRASODIUM EDTA, WATER
PRINCIPAL DISPLAY PANEL - 20 FL OZ bottle
#1 Doctor Recommended
Sensitive Skincare Brand
Cetaphil®
ACNE RELIEF
BODY WASH
Acne-Prone Skin
Maximum strength
OTC formula
to eliminate breakouts
24 hour dryness relief
Advanced Moisture Blend
2% Salicylic Acid
Acne Treatment
5 skin sensitivity signs
20 FL OZ (591 mL)
P55982-1