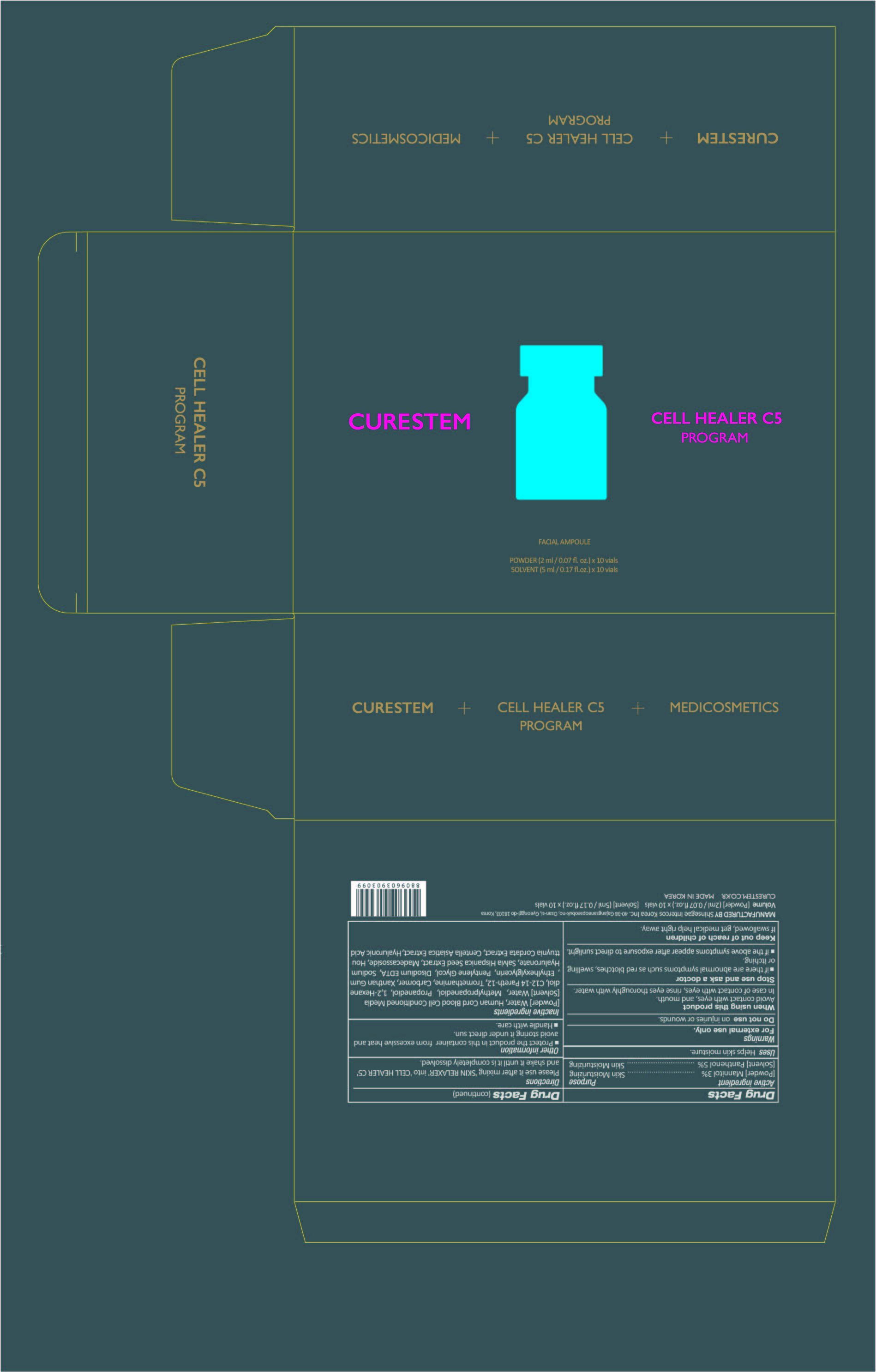
Label: CURESTEM CELL HEALER C5 PROGRAM- mannitol, panthenol kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 78521-100-01, 78521-100-10, 78521-200-01, 78521-200-10, view more78521-500-20 - Packager: DNK CORPORATION LTD.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 5, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children
- Directions
- Other information
-
Inactive ingredients
[Powder] Water, Human Cord Blood Cell Conditioned Media
[Solvent] Water, Methylpropanediol, Propanediol, 1,2-Hexanediol,
C12-14 Pareth-12, Tromethamine, Carbomer, Xanthan Gum,
Ethylhexylglycerin, Pentylene Glycol, Disodium EDTA, Sodium Hyaluronate,
Salvia Hispanica Seed Extract, Madecassoside, Houttuynia Cordata Extract,
Centella Asiatica Extract, Hyaluronic Acid
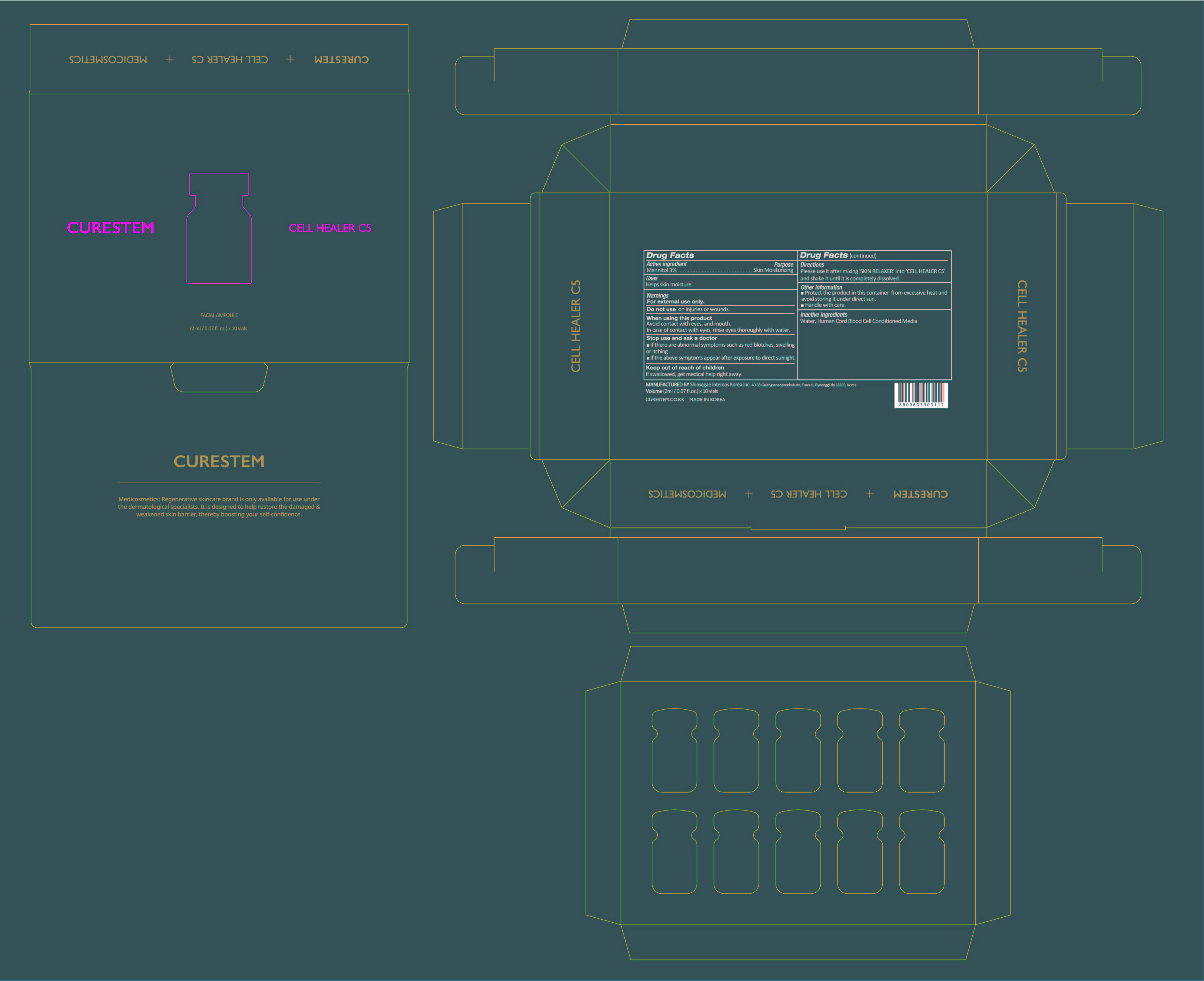
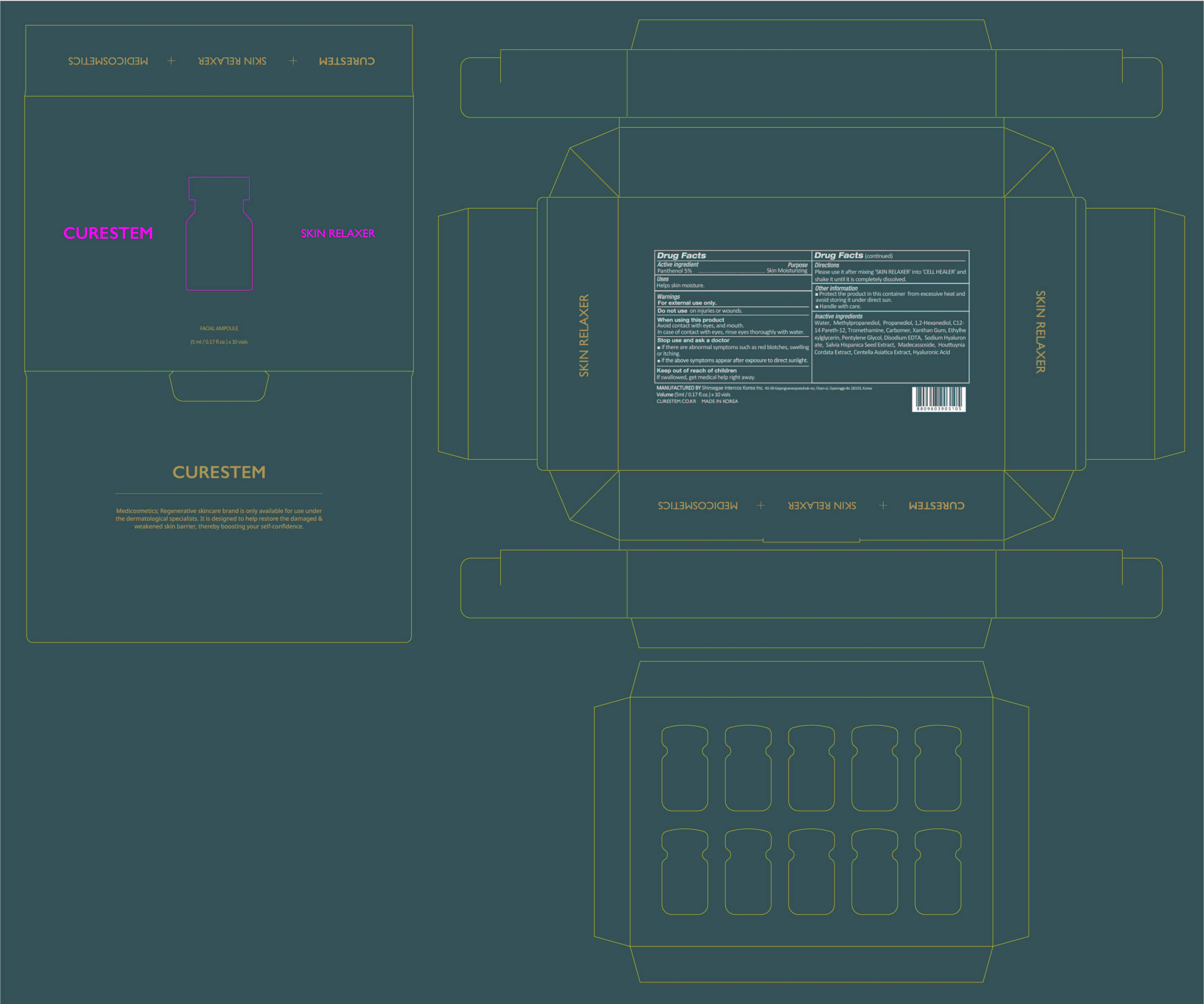
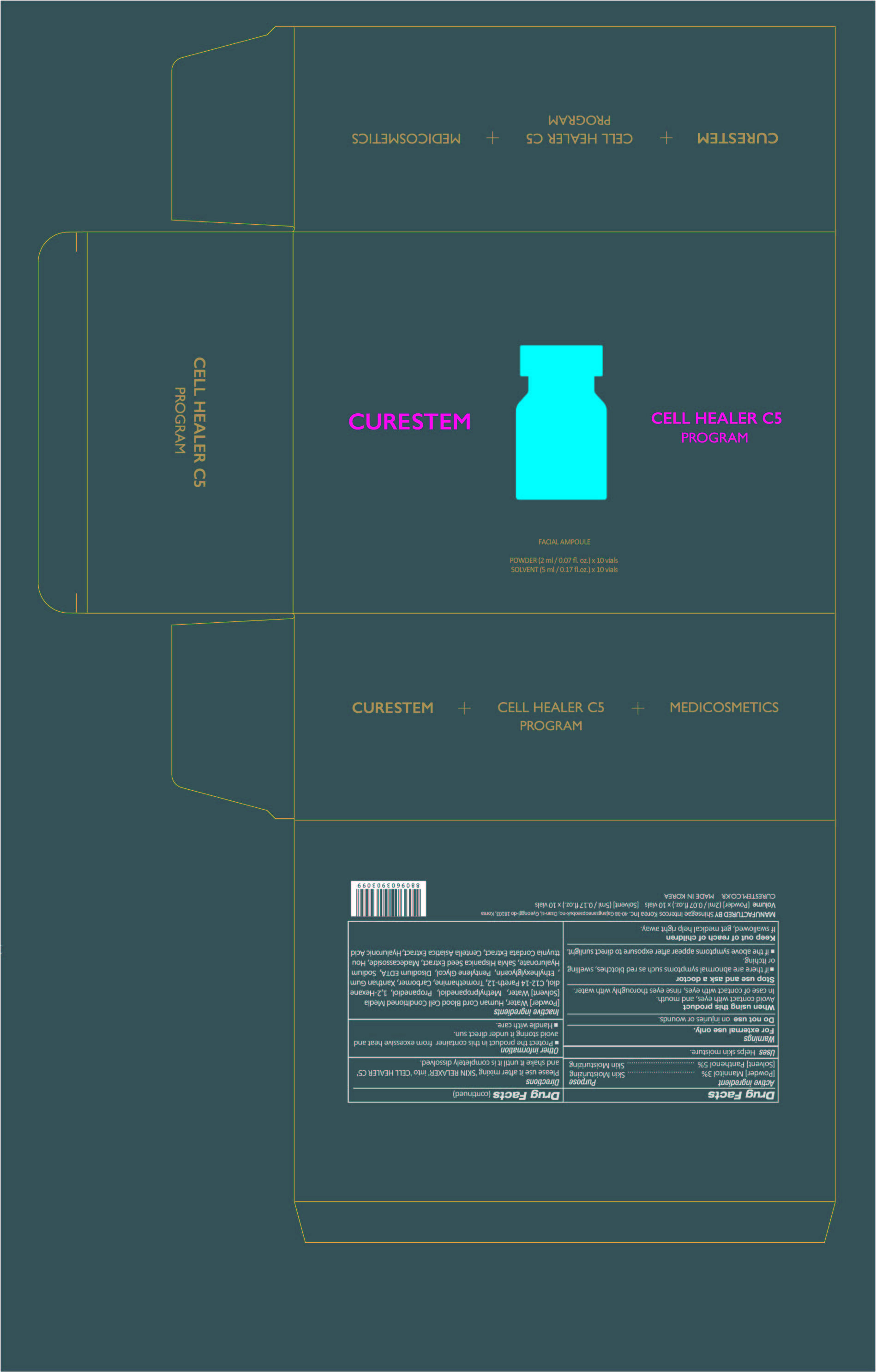
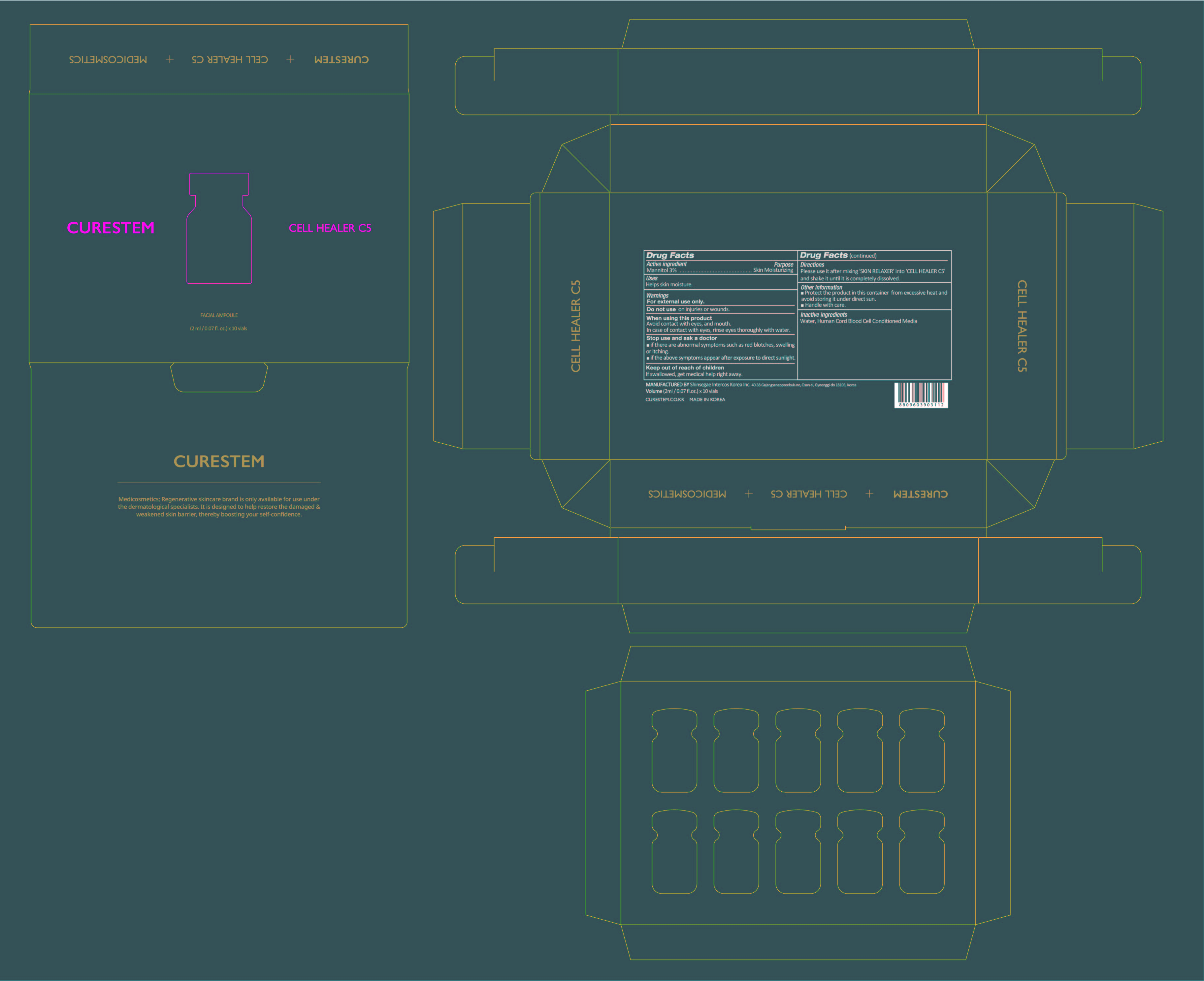
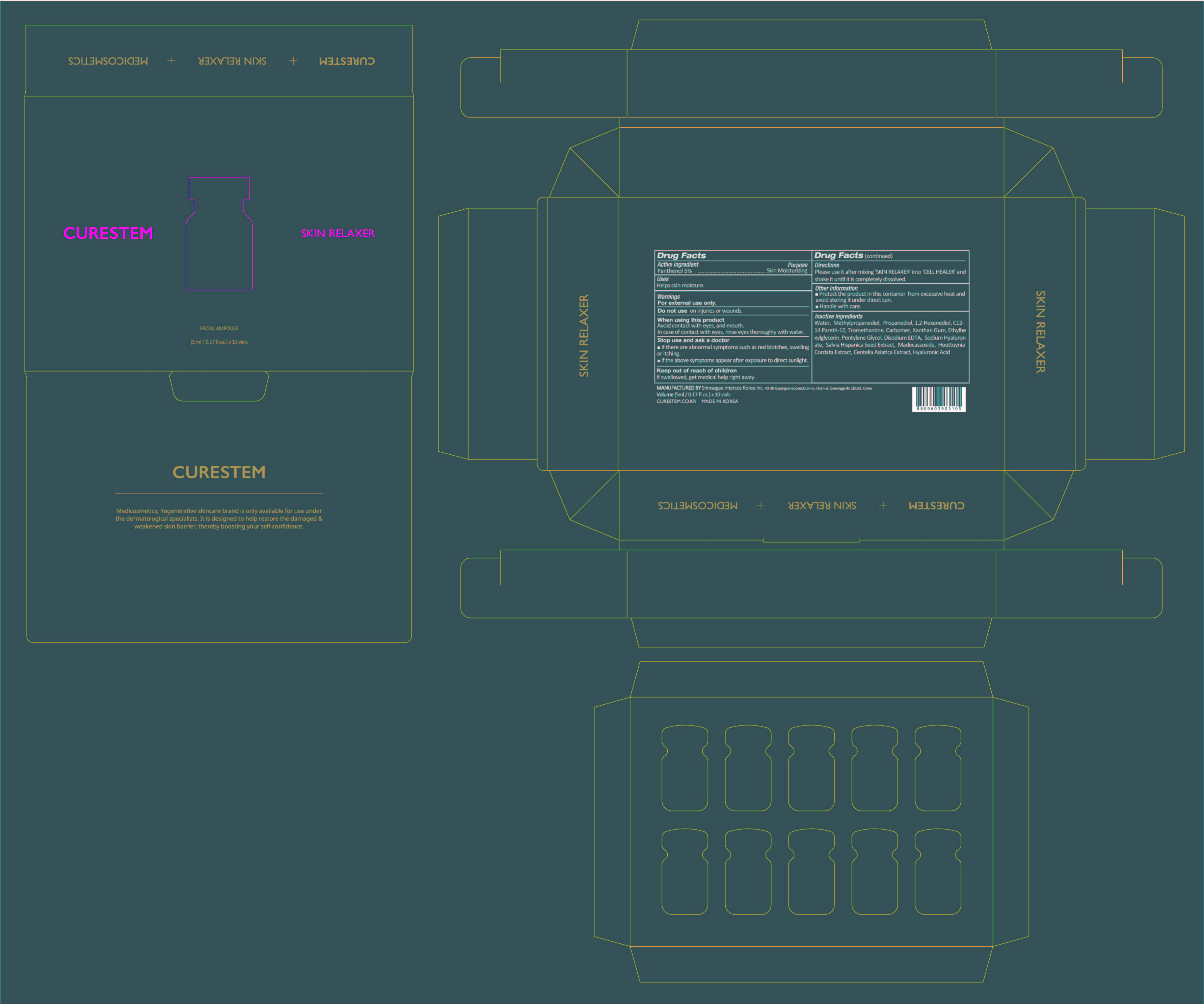
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CURESTEM CELL HEALER C5 PROGRAM
mannitol, panthenol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78521-500 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78521-500-20 1 in 1 BOX; Type 0: Not a Combination Product 06/05/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 2 mL Part 2 1 VIAL 5 mL Part 1 of 2 CURESTEM CELL HEALER C5
mannitol powderProduct Information Item Code (Source) NDC:78521-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 0.06 g in 2 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CORD BLOOD (UNII: PVH8394DLN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78521-100-10 10 in 1 BOX 1 NDC:78521-100-01 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/05/2020 Part 2 of 2 CURESTEM SKIN RELAXER
panthenol liquidProduct Information Item Code (Source) NDC:78521-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 0.25 g in 5 mL Inactive Ingredients Ingredient Name Strength METHYLPROPANEDIOL (UNII: N8F53B3R4R) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SALVIA HISPANICA SEED (UNII: NU0OLX06F8) CENTELLA ASIATICA (UNII: 7M867G6T1U) HYALURONIC ACID (UNII: S270N0TRQY) TROMETHAMINE (UNII: 023C2WHX2V) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PENTYLENE GLYCOL (UNII: 50C1307PZG) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) MADECASSOSIDE (UNII: CQ2F5O6YIY) PROPANEDIOL (UNII: 5965N8W85T) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) C12-14 PARETH-12 (UNII: M0LJS773XW) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78521-200-10 10 in 1 BOX 1 NDC:78521-200-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/05/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/05/2020 Labeler - DNK CORPORATION LTD. (695538778) Establishment Name Address ID/FEI Business Operations Shinsegae Intercos Korea Inc. 694526100 manufacture(78521-100, 78521-200, 78521-500)