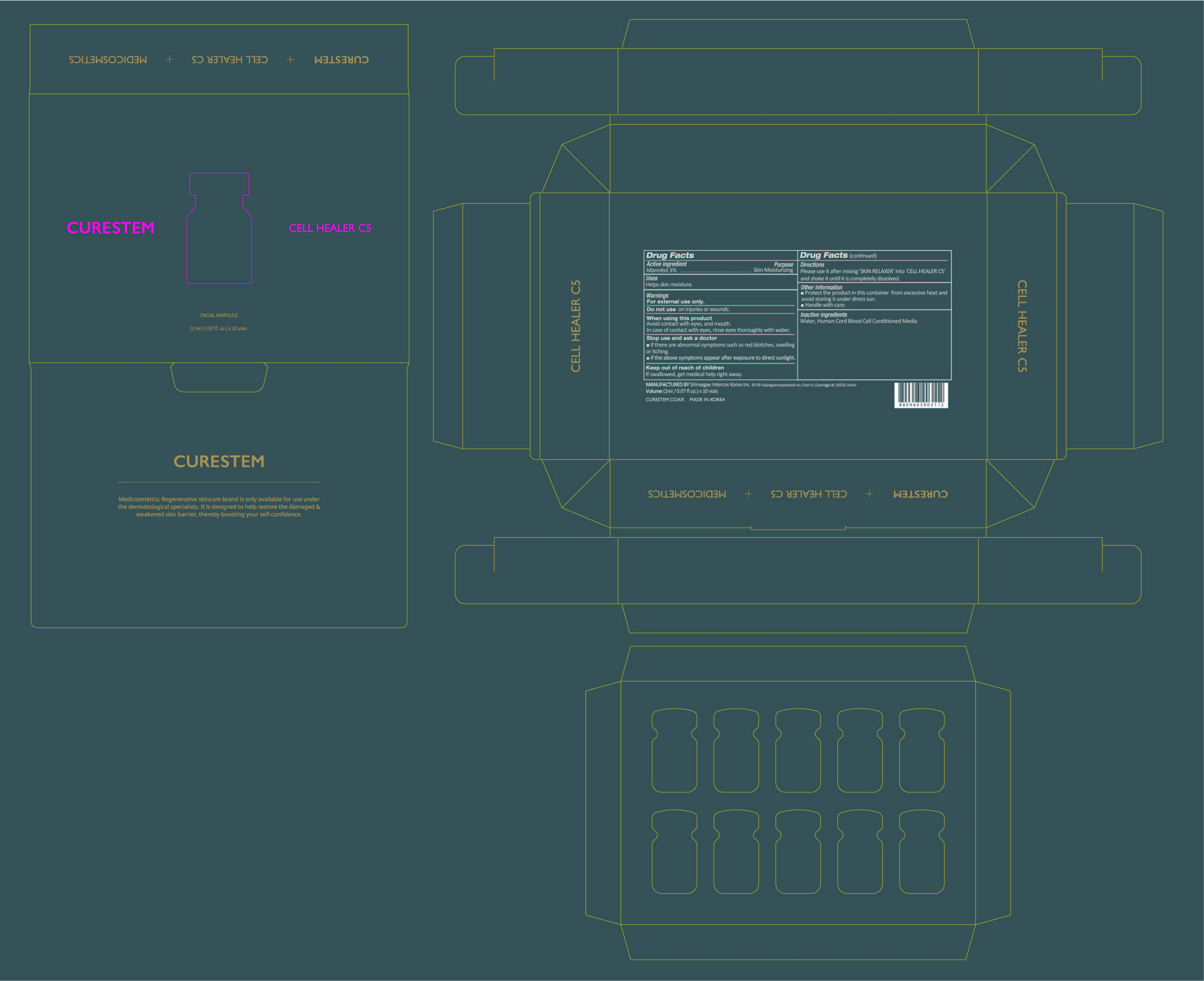
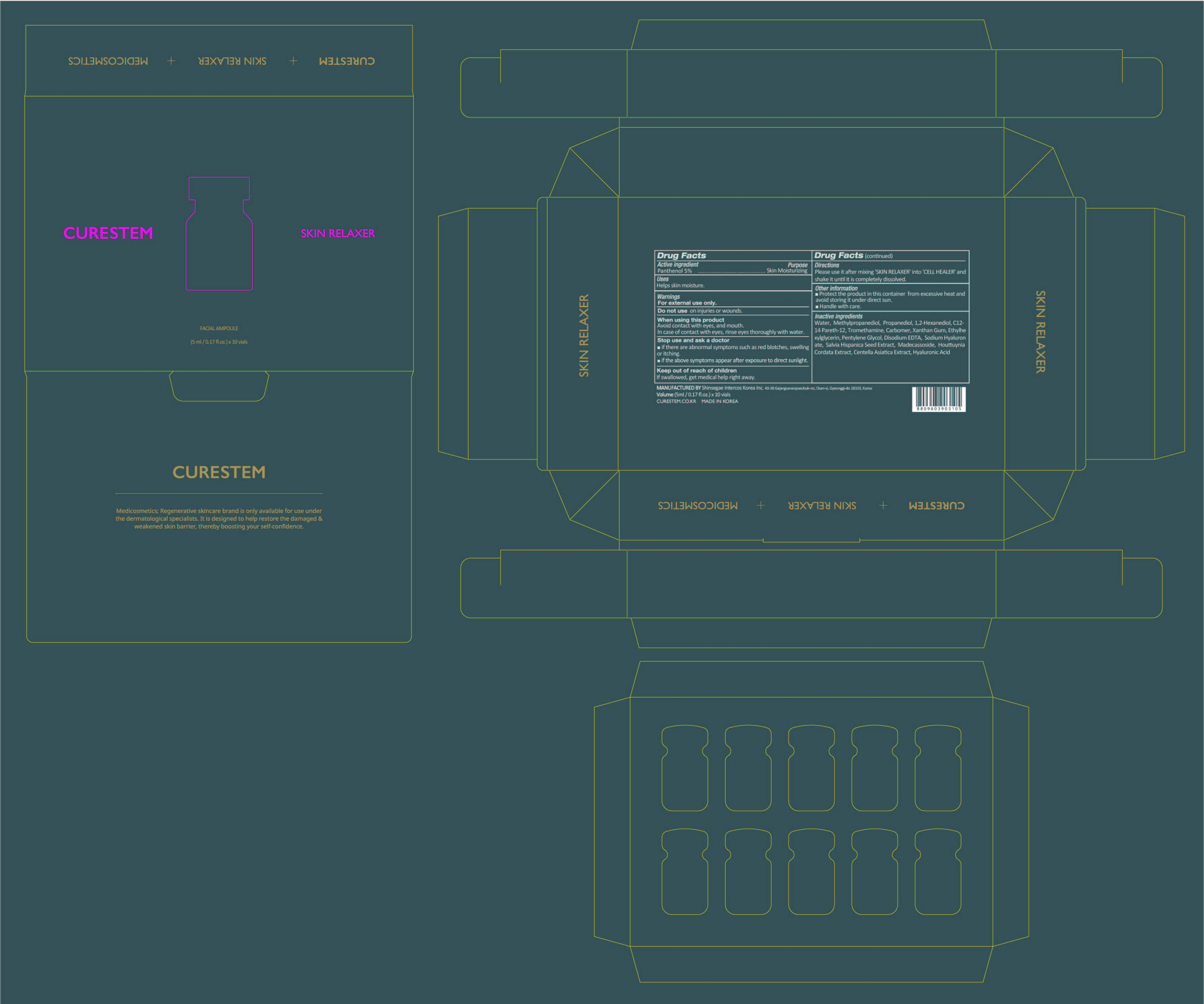
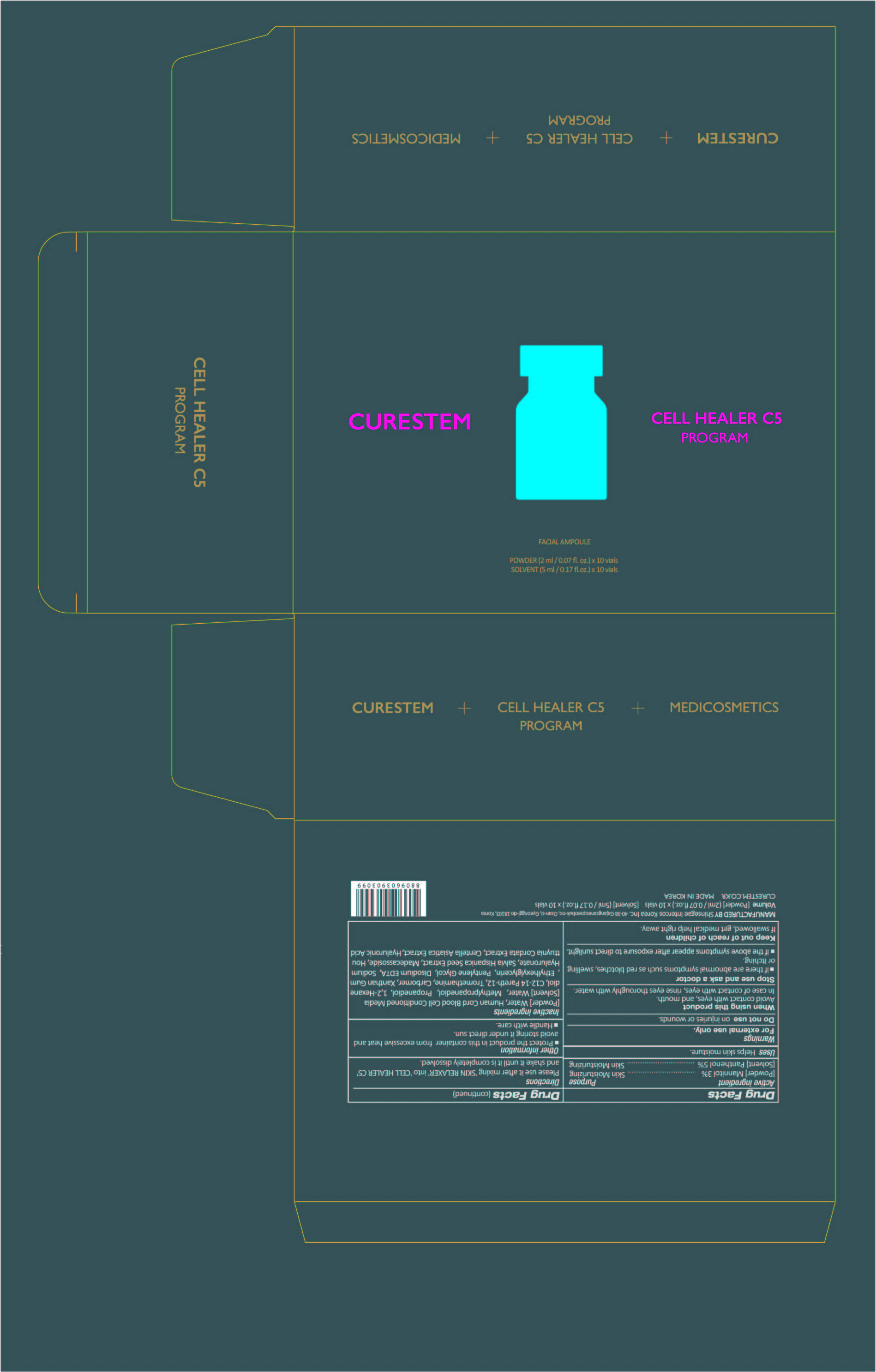
When using this product
Avoid contact with eyes, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor
■ if there are abnormal symptoms such as red blotches, swelling or itching.
■ if the above symptoms appear after exposure to direct sunlight.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Please use it after mixing 'SKIN RELAXER' into 'CELL HEALER C5'
and shake it until it is completely dissolved.
Other information
■ Protect the product in this container from excessive heat and
avoid storing it under direct sun.
■ Handle with care.
Inactive ingredients
[Powder] Water, Human Cord Blood Cell Conditioned Media
[Solvent] Water, Methylpropanediol, Propanediol, 1,2-Hexanediol,
C12-14 Pareth-12, Tromethamine, Carbomer, Xanthan Gum,
Ethylhexylglycerin, Pentylene Glycol, Disodium EDTA, Sodium Hyaluronate,
Salvia Hispanica Seed Extract, Madecassoside, Houttuynia Cordata Extract,
Centella Asiatica Extract, Hyaluronic Acid