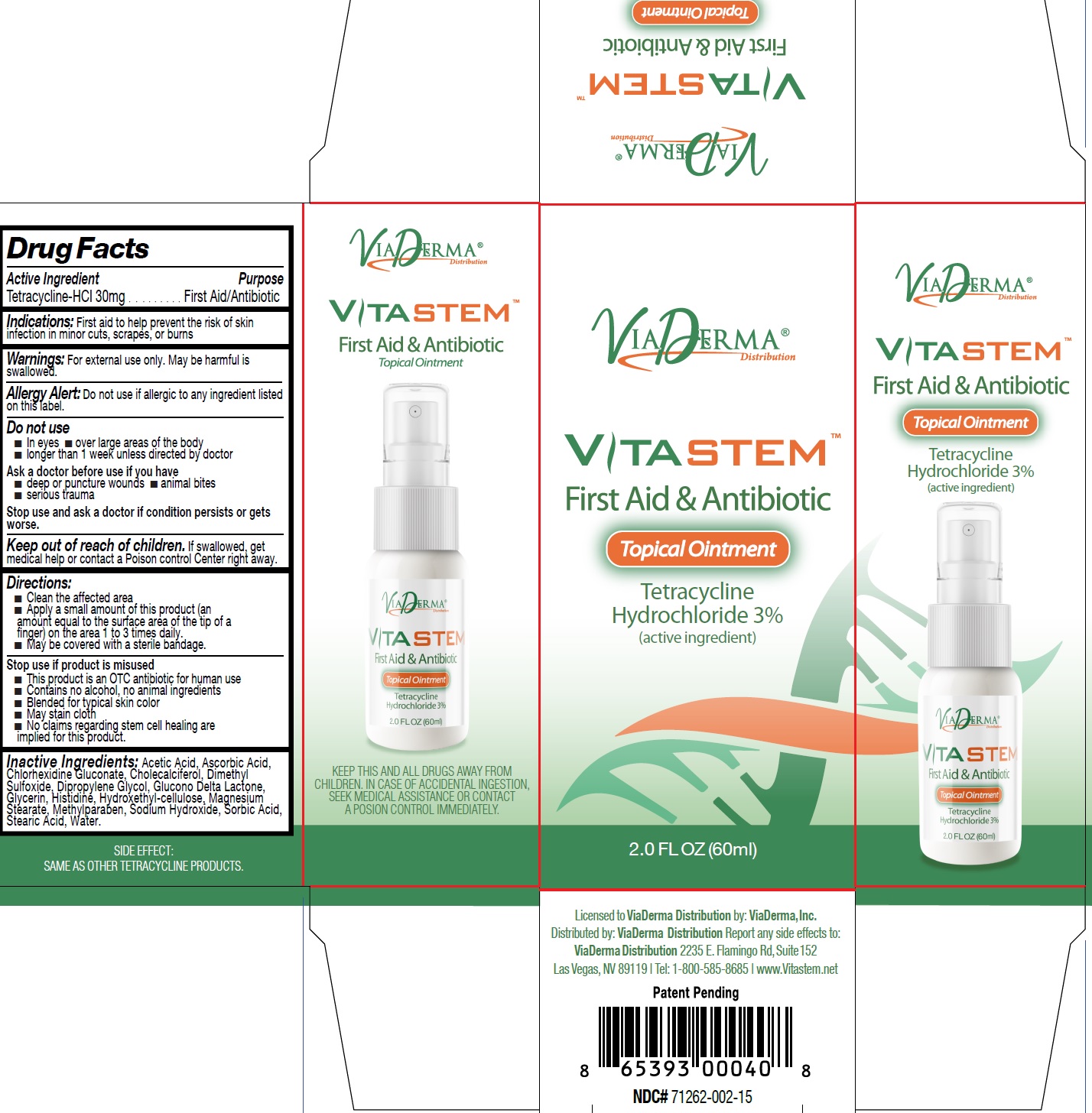
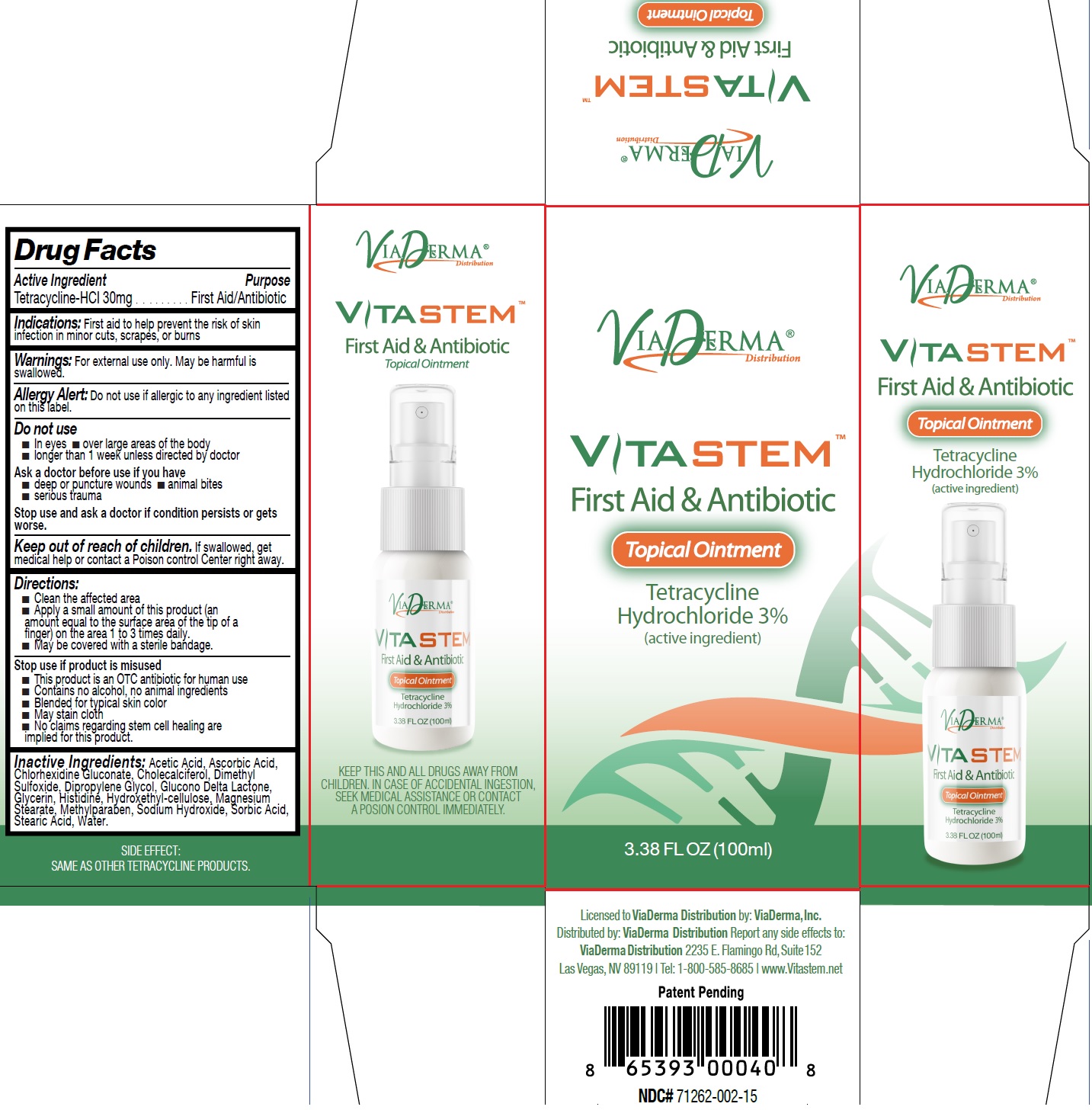
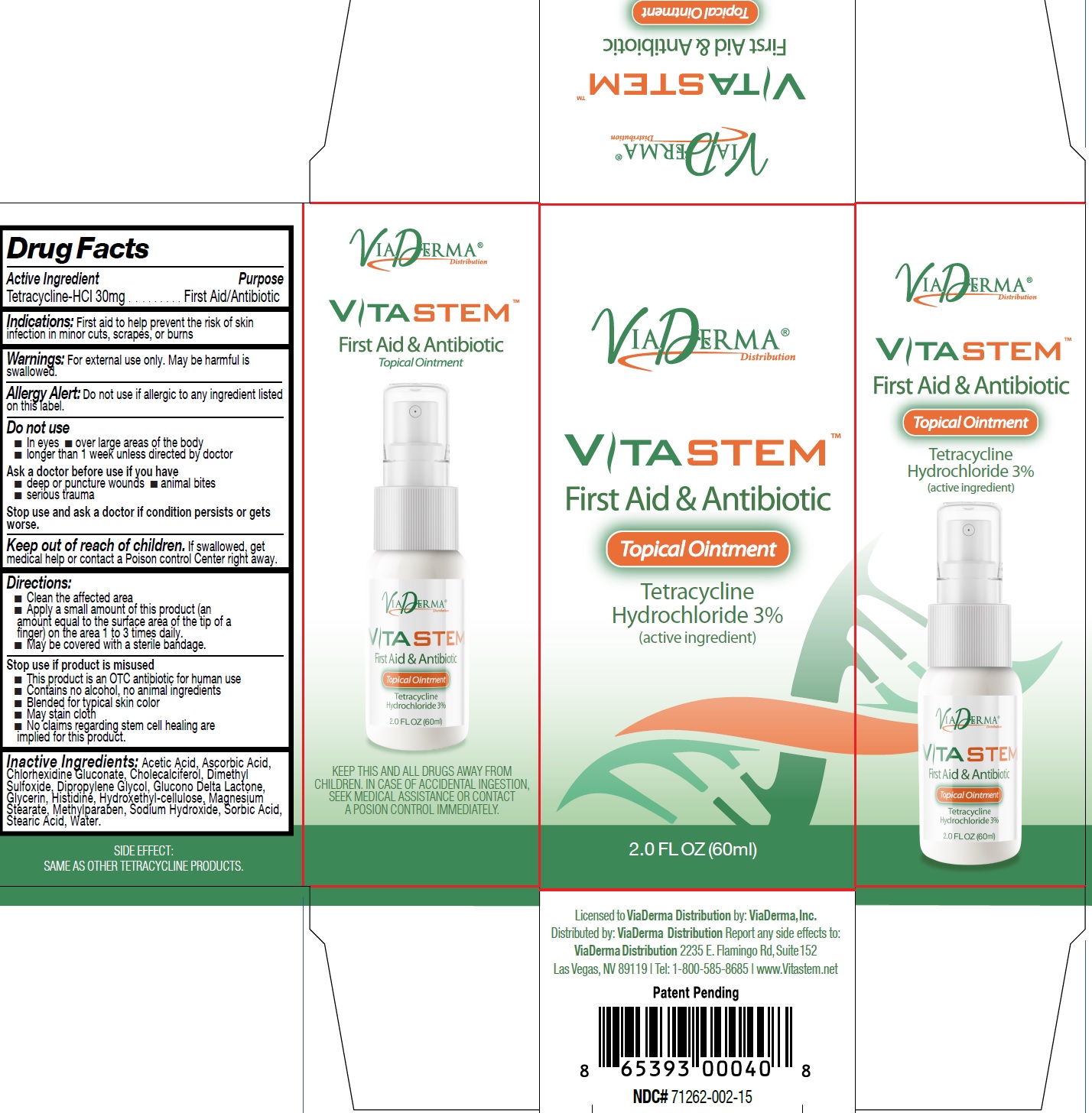
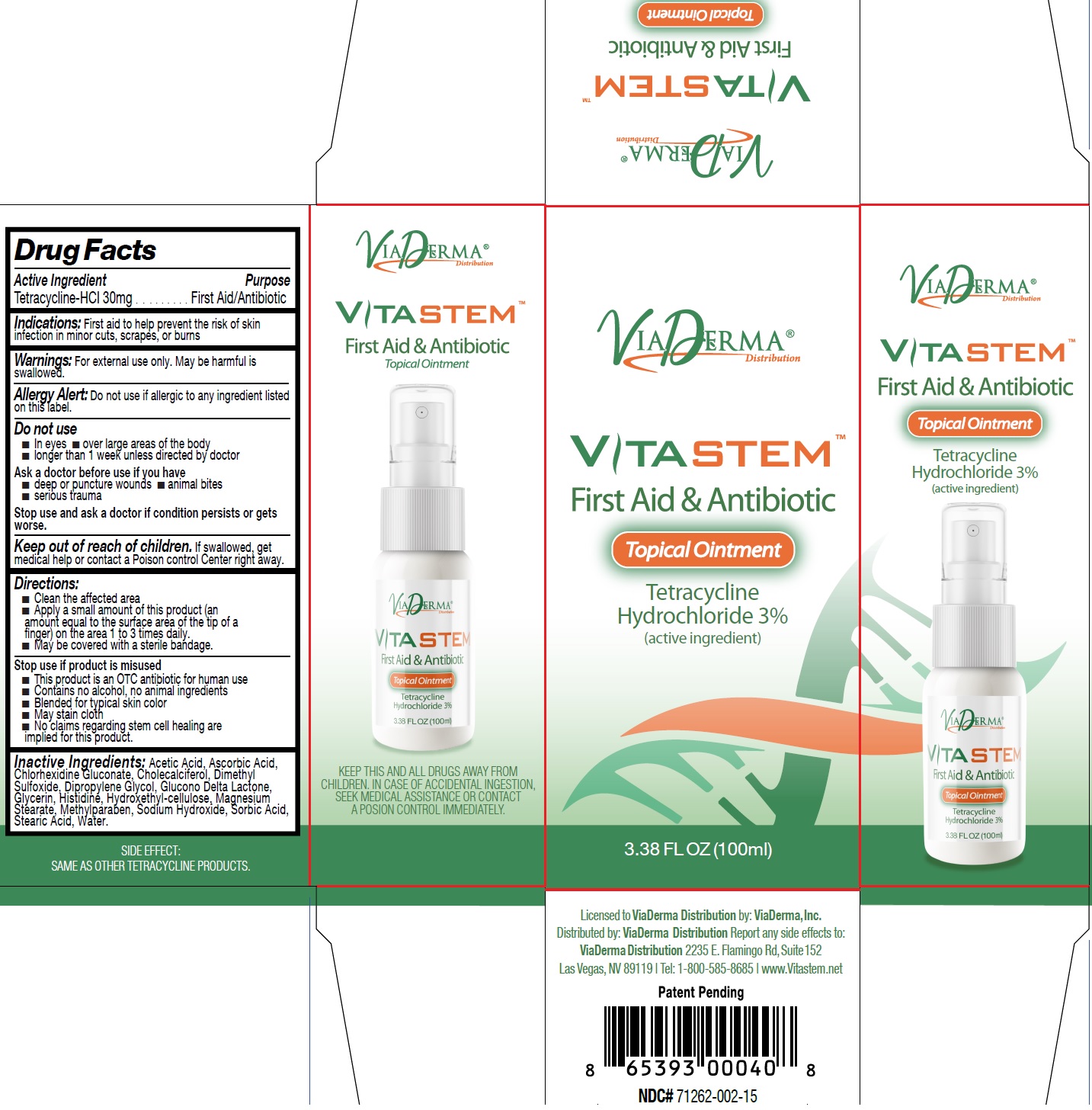
Label: VITASTEM- tetracycline hydrochloride ointment
- NDC Code(s): 71262-002-00, 71262-002-01, 71262-002-02, 71262-002-15
- Packager: ViaDerma Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Indications:
- Warnings:
-
Directions:
- Clean the affected area
- Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily.
- May be covered with a sterile bandage.
- This product is an OTC antibiotic for human use
- Contains no alcohol, no animal ingredients
- Blended for typical skin color
- May stain cloth
- No claims regarding stem cell healing are implied for this product.
Stop use if product is misused
- Inactive Ingredients:
- Package Labeling:
- VITASTEM-tetracycline hydrochloride ointment, 60 ml (71262-002-01)
- VITASTEM-tetracycline hydrochloride ointment, 100 ml (71262-002-02
-
INGREDIENTS AND APPEARANCE
VITASTEM
tetracycline hydrochloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71262-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACYCLINE HYDROCHLORIDE (UNII: P6R62377KV) (TETRACYCLINE - UNII:F8VB5M810T) TETRACYCLINE HYDROCHLORIDE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) ASCORBIC ACID (UNII: PQ6CK8PD0R) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) CHOLECALCIFEROL (UNII: 1C6V77QF41) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) HISTIDINE (UNII: 4QD397987E) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBIC ACID (UNII: X045WJ989B) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71262-002-00 1 in 1 BOX 02/09/2017 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:71262-002-15 1 in 1 BOX 07/01/2017 2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:71262-002-01 1 in 1 BOX 11/08/2016 3 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:71262-002-02 1 in 1 BOX 11/08/2016 4 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 11/08/2016 Labeler - ViaDerma Distribution, Inc. (081113521)