Active Ingredient
Tetracycline-HCI 30mg
Purpose
First Aid/Antibiotic
Indications:
First aid to help prevent the risk of skin infection in minor cuts, scrapes, or burns
Warnings:
For external use only. May be harmful is swallowed.
Do not use if allergic to any ingredient listed on this label.
Allergy Alert:
Do not use
- In eyes
- over large areas of the body
- longer than 1 week unless directed by doctor
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious trauma
Stop use and ask a doctor
if condition persists or gets worse.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison control Center right away.
Directions:
- Clean the affected area
- Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily.
- May be covered with a sterile bandage.
- This product is an OTC antibiotic for human use
- Contains no alcohol, no animal ingredients
- Blended for typical skin color
- May stain cloth
- No claims regarding stem cell healing are implied for this product.
Stop use if product is misused
Inactive Ingredients:
Acetic Acid, Ascorbic Acid, Chlorhexidine Gluconate, Cholecalciferol, Dimethyl Sulfoxide, Dipropylene Glycol, Glucono Delta Lactone, Glycerin, Histidine, Hydroxethyl-cellulose, Magnesium Stearate, Methylparaben, Sodium Hydroxide, Sorbic Acid, Stearic Acid, Water.
Package Labeling:

VITASTEM-tetracycline hydrochloride ointment, 60 ml (71262-002-01)
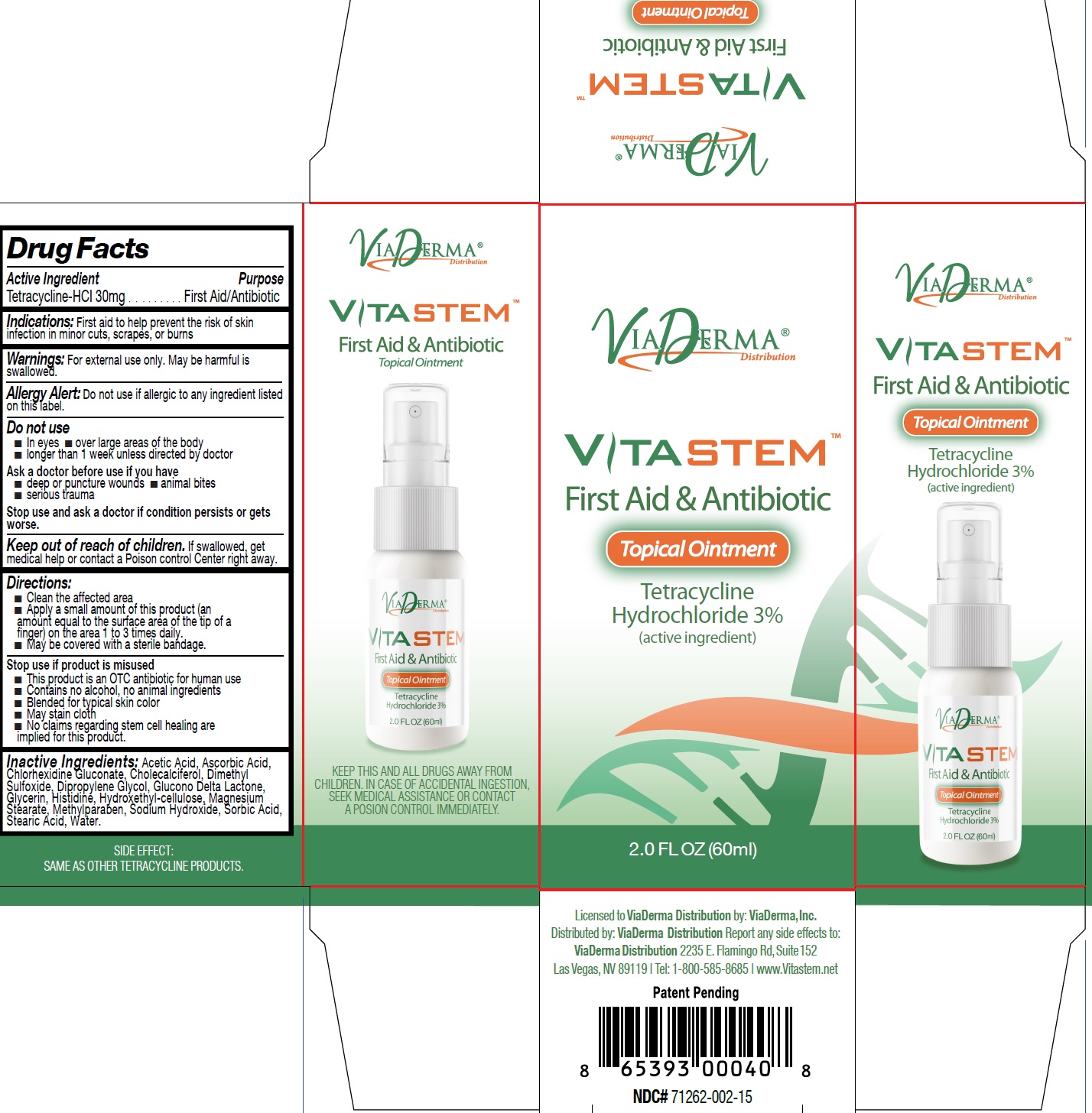
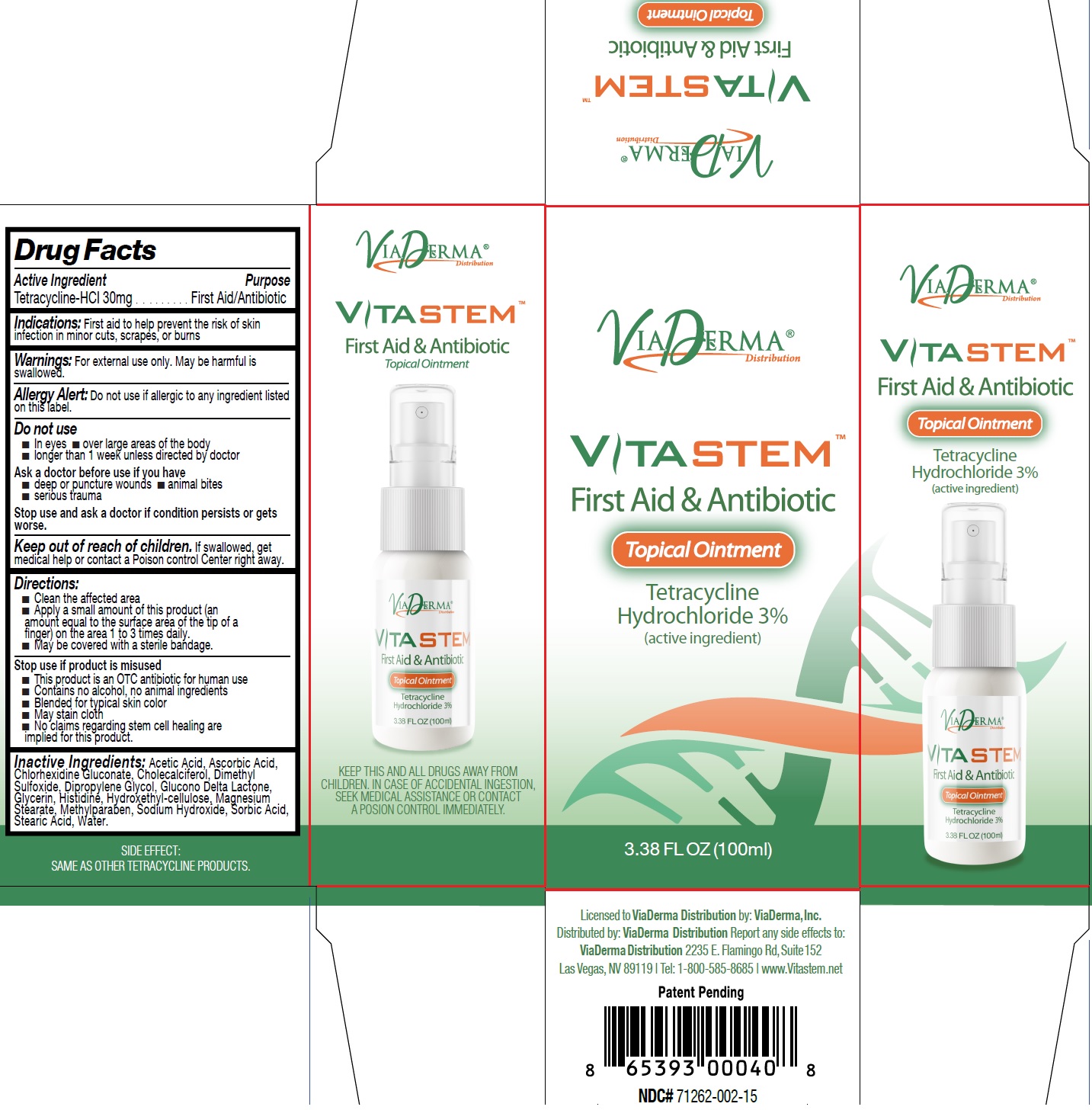
VITASTEM-tetracycline hydrochloride ointment, 100 ml (71262-002-02

ViaDerma Distribution, Inc.



