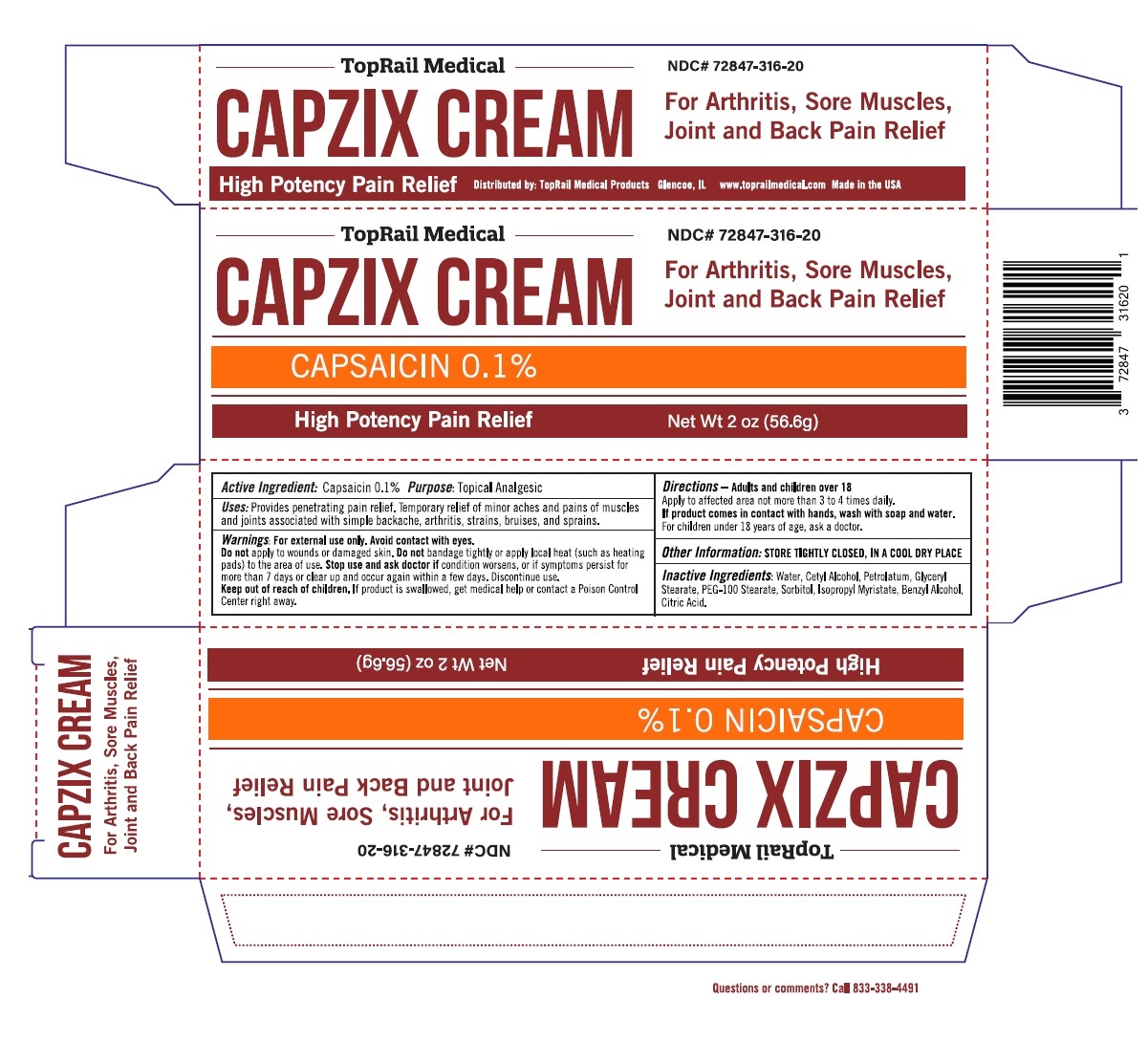
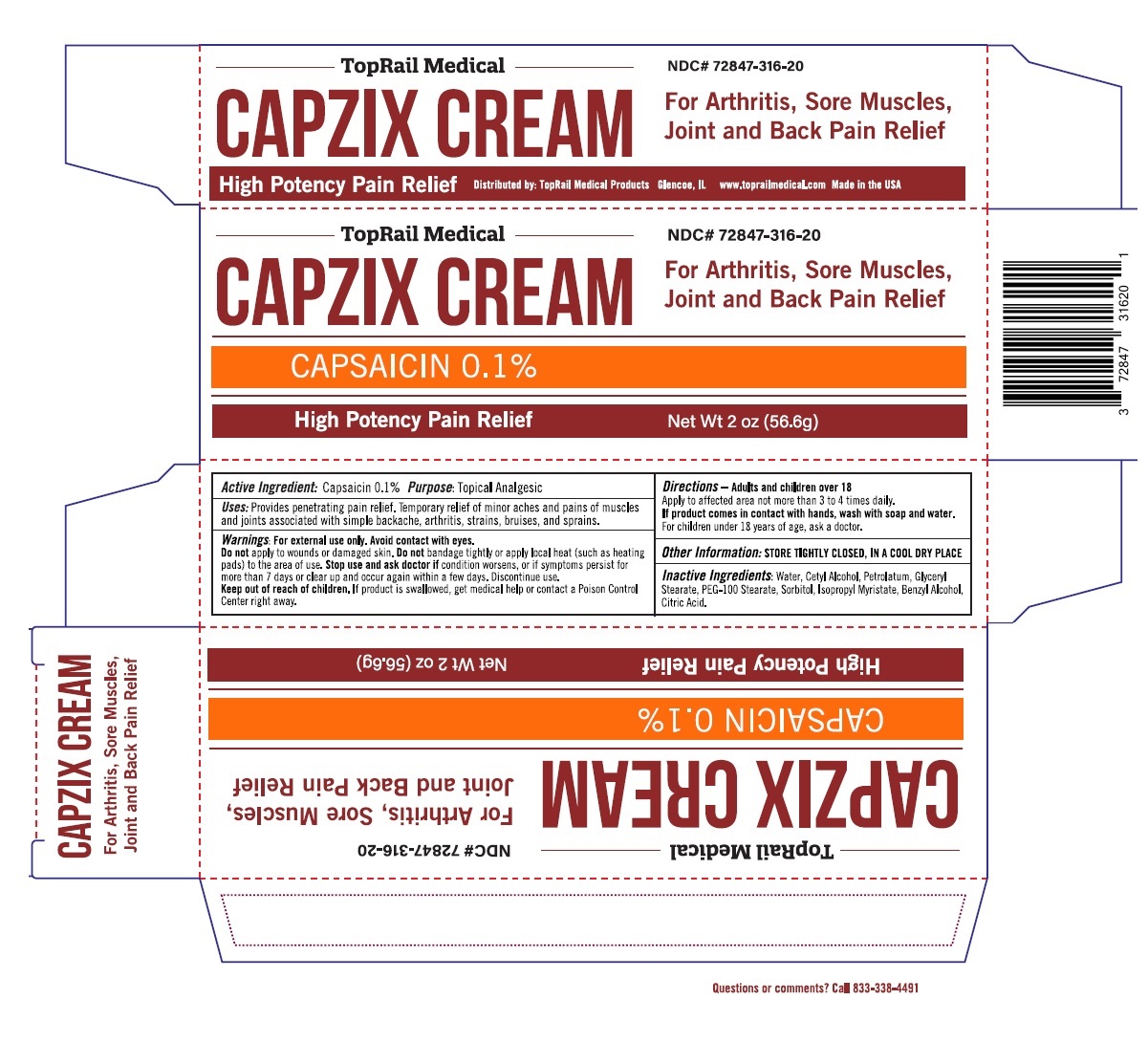
Label: CAPZIX- capsaicin cream
- NDC Code(s): 72847-316-20
- Packager: Toprail Chp Llc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 30, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings: For external use only. Avoid contact with eyes.
Do not apply to wounds or damaged skin. Do not bandage tightly or apply local heat (such as heating pads) to the area of use. Stop use and ask doctor if condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days. Discontinue use. - DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
CAPZIX
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72847-316 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.001 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETYL ALCOHOL (UNII: 936JST6JCN) PETROLATUM (UNII: 4T6H12BN9U) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) SORBITOL (UNII: 506T60A25R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) BENZYL ALCOHOL (UNII: LKG8494WBH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72847-316-20 1 in 1 CARTON 07/19/2019 1 56.6 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/19/2019 Labeler - Toprail Chp Llc. (080783804)