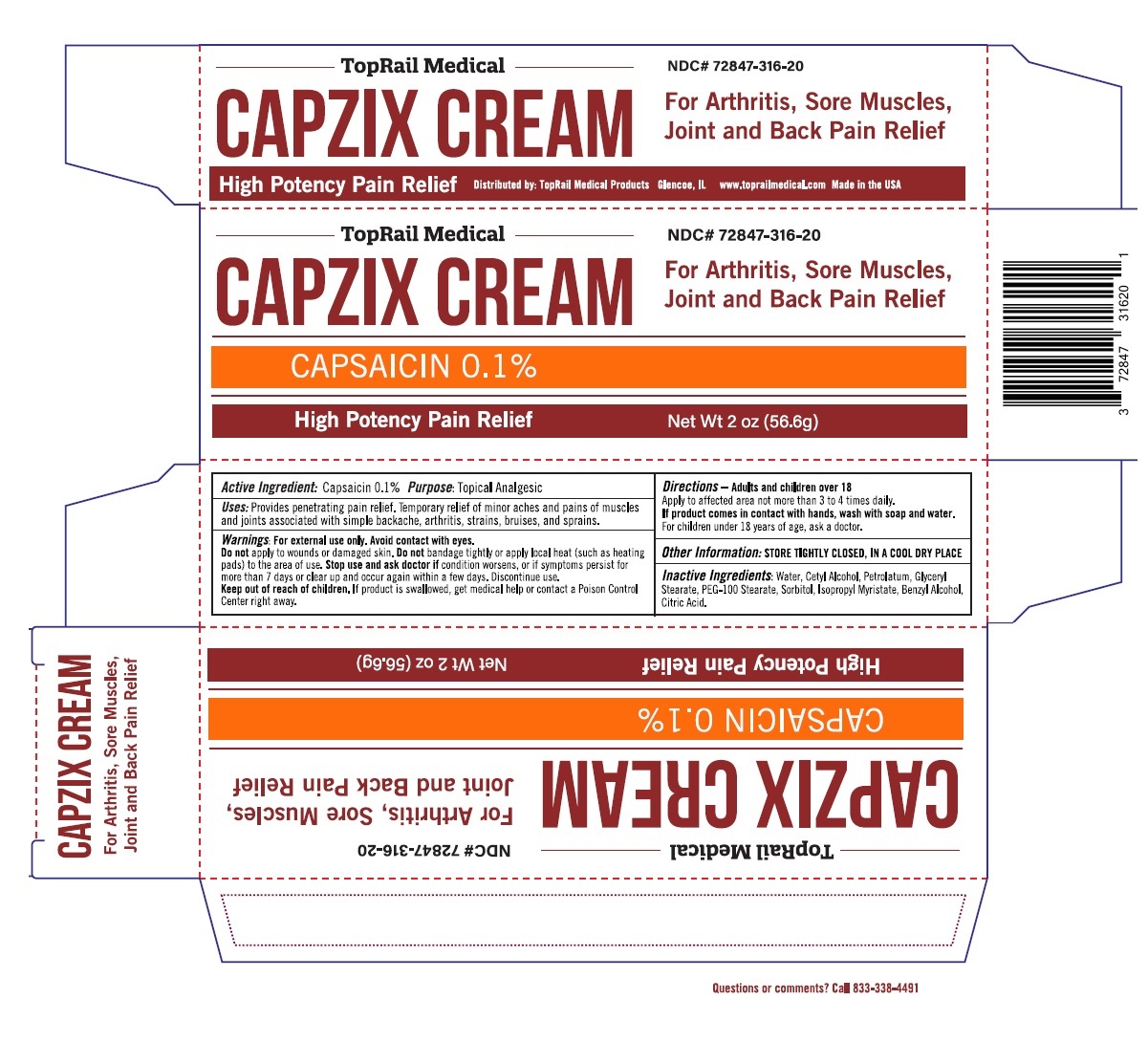
Uses: Provides penetrating pain relief. Temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises, and sprains.
Warnings: For external use only. Avoid contact with eyes.
Do not apply to wounds or damaged skin. Do not bandage tightly or apply local heat (such as heating pads) to the area of use. Stop use and ask doctor if condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days. Discontinue use.
Directions – Adults and children over 18
Apply to affected area not more than 3 to 4 times daily.
If product comes in contact with hands, wash with soap and water.
For children under 18 years of age ask a doctor.
Inactive Ingredients: Water, Cetyl Alcohol, Petrolatum, Glyceryl Stearate, PEG-100 Stearate, Sorbitol, Isopropyl Myristate, Benzyl Alcohol, Citric Acid.