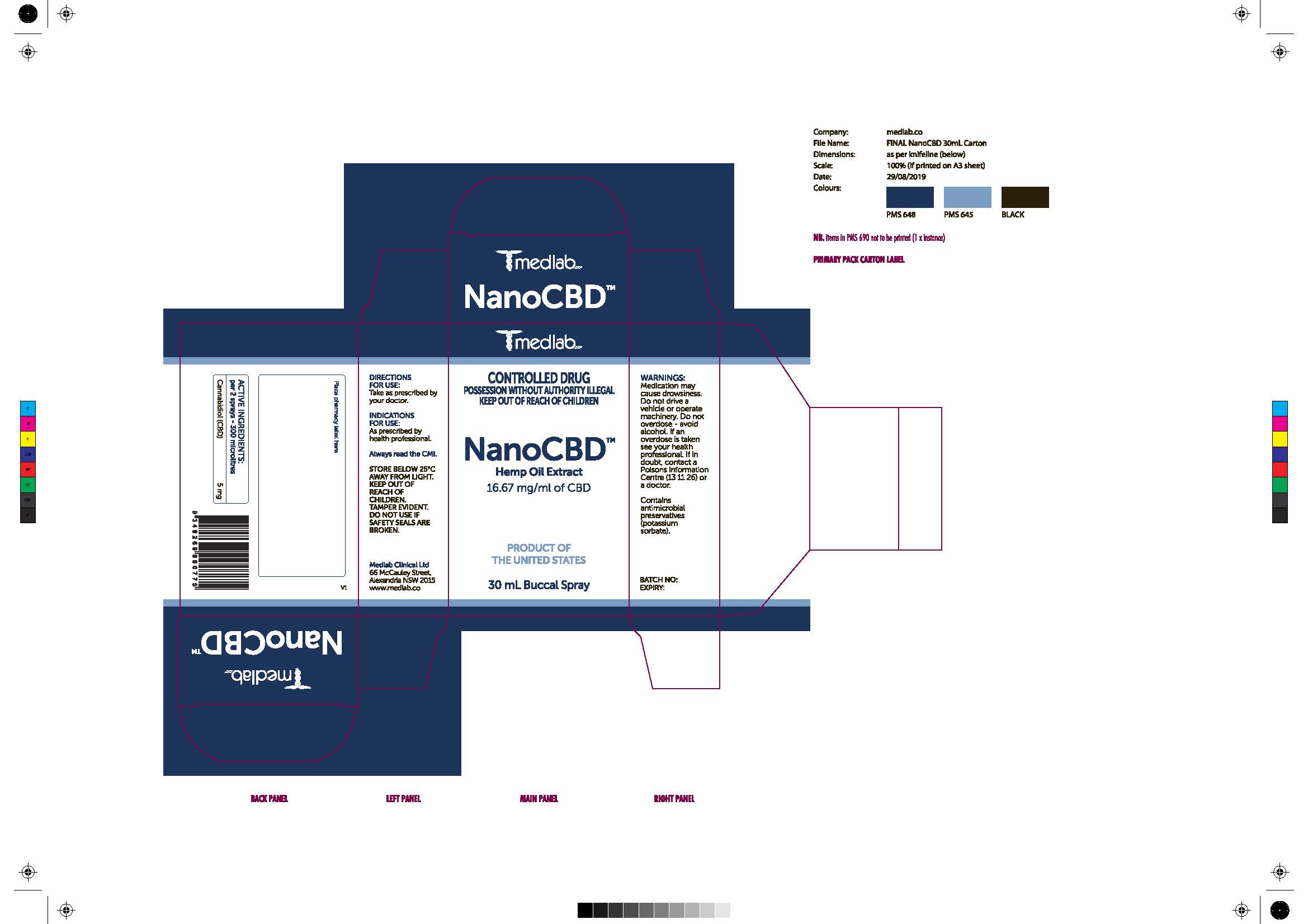
Label: NANOCDB- none solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 70658-122-12 - Packager: Creative Essences Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated April 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warning Section (Carton AU)
- Carton Food Safety Warning
- Indication for Use:
- Active ingredients and direction (Carton AU)
- Other Safety Information on Cartons (AU)
- Inactive Ingredients on Leaflet (Page 2)
-
Section 8 of Leaflet
What is looks like
NanoCBD is provided as a dark-amber liquid in a while 30 mL plastic spray container and a pump. The pump is protected with a plastic cup. NanoCelle is a novel drug delivery system that enables very small particles to be delivered as an oral spray. The Nanocelle spray contains submicron size particles with a fat-soluble core containing the drug, and a water-soluble outer layer.
NanoCBD is a mouth spray (oro-buccal spray) wihch contains the active ingredient cannacidiol.

- Dosage and Administration on Product Label
- How to take NanoCBD (page 1 Leaflet)
- PDP
-
INGREDIENTS AND APPEARANCE
NANOCDB
none solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70658-122 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANNABIDIOL (UNII: 19GBJ60SN5) (CANNABIDIOL - UNII:19GBJ60SN5) CANNABIDIOL 1.938 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 77.27 g in 100 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.096 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.41 g in 100 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 0.1 g in 100 mL REBAUDIOSIDE A (UNII: B3FUD0528F) PEPPERMINT OIL (UNII: AV092KU4JH) 0.31 g in 100 mL POLYOXYL 40 CASTOR OIL (UNII: 4ERD2076EF) 15.65 g in 100 mL Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70658-122-12 1 in 1 CARTON 04/21/2020 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 04/21/2020 Labeler - Creative Essences Inc (079120182) Registrant - Creative Essences Inc (079120182) Establishment Name Address ID/FEI Business Operations Creative Essences Inc 079120182 manufacture(70658-122)