Warning Section (Carton AU)
Medication may cause drowsiness. Do not drive a vehicle or operate machinery. Do not overdose - avoid alcohol. If an overdoes is taken see your health professional. If in doubt, contact a Poisons Information Centre (13 11 26) or a doctor.
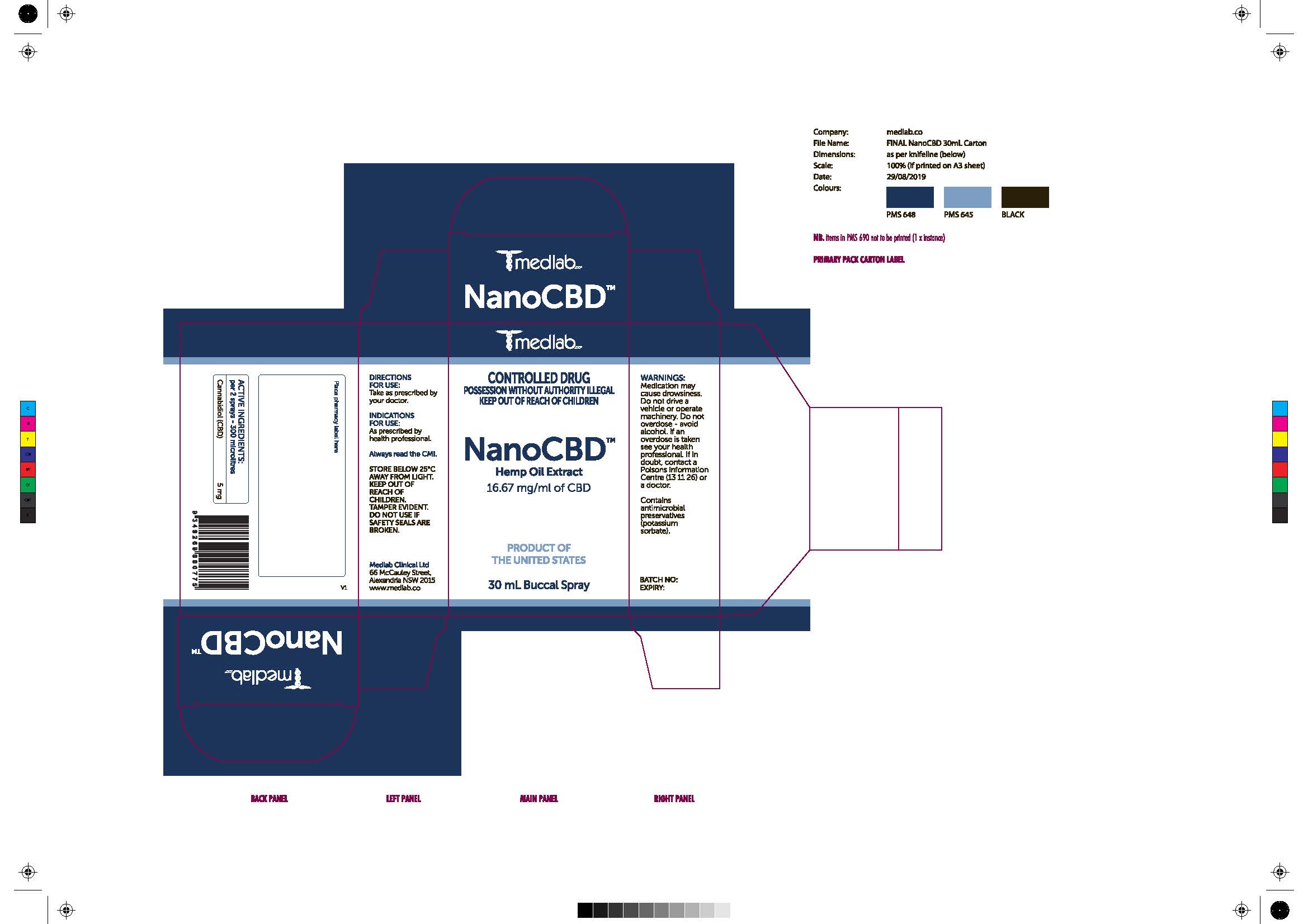
Other Safety Information on Cartons (AU)
Store below 25C away from light. Keep out of reach of children. Tamper Evident. Do not use if safety seals are broken.
Inactive Ingredients on Leaflet (Page 2)
Water, modified vegetable oil, glycerol, citric acid, potassium sorbate, sucralose, peppermint oil
Section 8 of Leaflet
What is looks like
NanoCBD is provided as a dark-amber liquid in a while 30 mL plastic spray container and a pump. The pump is protected with a plastic cup. NanoCelle is a novel drug delivery system that enables very small particles to be delivered as an oral spray. The Nanocelle spray contains submicron size particles with a fat-soluble core containing the drug, and a water-soluble outer layer.
NanoCBD is a mouth spray (oro-buccal spray) wihch contains the active ingredient cannacidiol.


