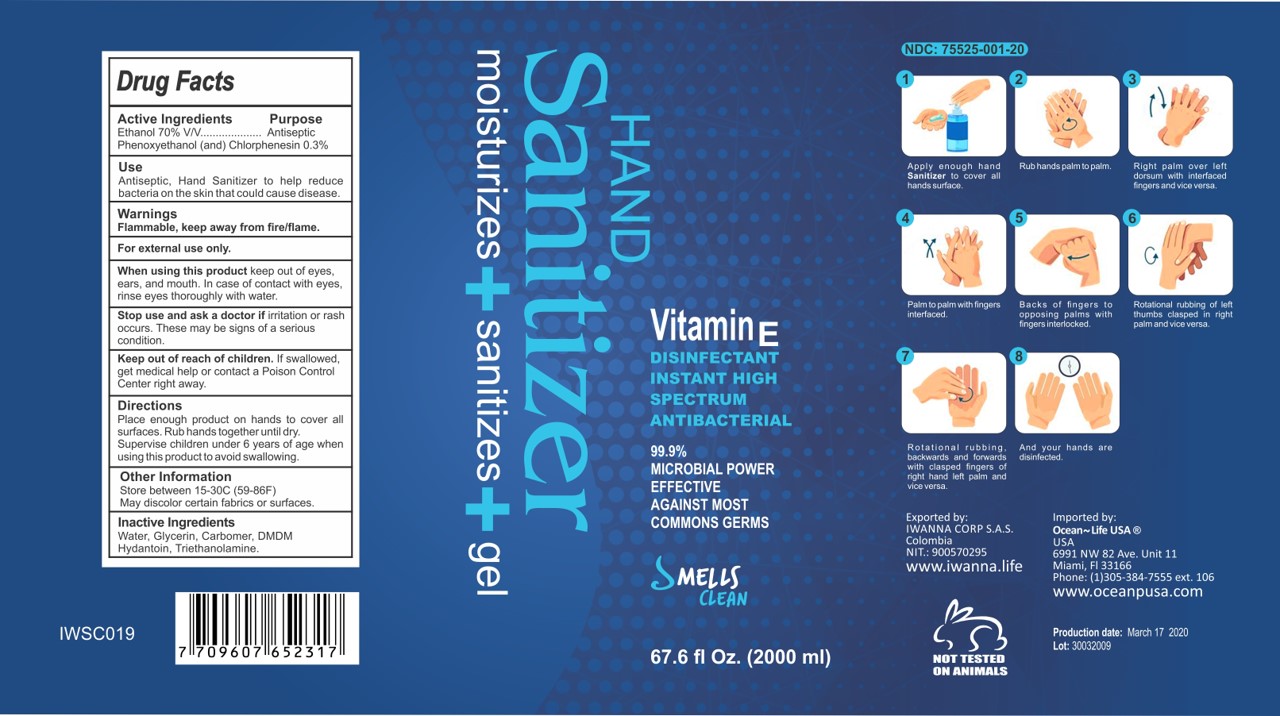
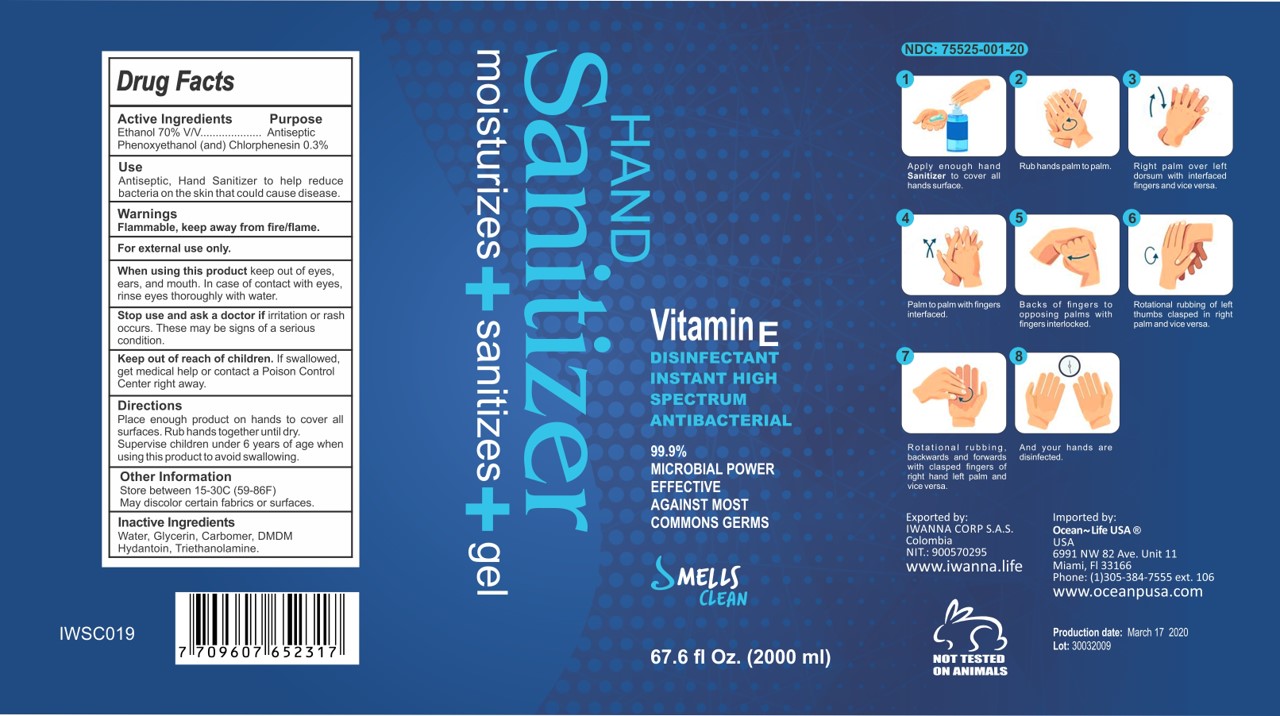
Label: HAND SANITIZER SMELLS CLEAN- ethanol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 75525-001-06, 75525-001-07, 75525-001-10, 75525-001-11, view more75525-001-12, 75525-001-20, 75525-001-21, 75525-001-25, 75525-001-40, 75525-001-50, 75525-001-51 - Packager: IWANNA CORP SAS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HAND SANITIZER
This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (70%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (1.00% v/v).
- Phenoxyethanol and chlorphenesin (0.3% v/v).
- Sterile distilled water or boiled cold water.
- Active Ingredient(s)
- Purpose
-
Indications and Usage
Apply enough hand Sanitizer to cover all hands surface
Rub hands palm to palm
Right palm over left dorsum with interfaced fingers and vice versa.
Palm to palm with fingers interfaced.
Backs of fingers to opposing palms with fingers interlocked.
Rotational rubbing of left thumbs clasped in right palmed and vice versa.
Rotational rubbing backwards and forwards with clasped fingers of right hand and left palm and vice versa.
And your hands are disinfected. -
Warnings
Flammable. Keep away from fire or flame
For external use only.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- Directions
- KEEP OUT OF REACH OF CHILDREN
- Other information
- Inactive ingredients
-
Package Label - Principal Display Panel
Hand Sanitizer
moisturizes+sanitizes+gel
Hand Sanitizer- 500 ML
Hand Sanitizer
moisturizes+sanitizes+gel
16.9 fl. oz (500 mL) NDC: 75525-001-50

HAND SANITIZER 1000 ML
Hand Sanitizer
moisturizes+sanitizes+gel
33.8 fl. oz (1000 mL) NDC: 75525-001-10

HAND SANITIZER 2000 ML
Hand Sanitizer
moisturizes+sanitizes+gel
67.6 fl. oz (2000 mL) NDC: 75525-001-20
HAND SANITIZER 120 ML
Hand Sanitizer
moisturizes+sanitizes+gel
4.0 fl. oz (120 mL) NDC: 75525-001-12

HAND SANITIZER 250 ML
Hand Sanitizer
moisturizes+sanitizes+gel
8.4 fl. oz (250 mL) NDC: 75525-001-25

HAND SANITIZER 60 ML-DISPENSER
Hand Sanitizer
moisturizes+sanitizes+gel
2.02 FL OZ (60 ML)
NDC:75525-001-07

HAND SANITIZER 2000 ML DISP
Hand Sanitizer
moisturizes+sanitizes+gel
67.6 fl. oz (2000 mL) NDC: 75525-001-21

HAND SANITIZER 3875 ML DISP
Hand Sanitizer
moisturizes+sanitizes+gel
131 fl. oz (3875 mL) NDC: 75525-001-40

-
INGREDIENTS AND APPEARANCE
HAND SANITIZER SMELLS CLEAN
ethanol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75525-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENESIN (UNII: I670DAL4SZ) (CHLORPHENESIN - UNII:I670DAL4SZ) CHLORPHENESIN 0.143 g in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) (PHENOXYETHANOL - UNII:HIE492ZZ3T) PHENOXYETHANOL 0.143 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER 940 (UNII: 4Q93RCW27E) 0.2685 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 0.95 g in 100 mL WATER (UNII: 059QF0KO0R) 27.8 mL in 100 mL .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.009 g in 100 mL TROLAMINE (UNII: 9O3K93S3TK) 0.479 g in 100 mL DMDM HYDANTOIN (UNII: BYR0546TOW) 0.095 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75525-001-06 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/30/2020 2 NDC:75525-001-50 500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/30/2020 3 NDC:75525-001-10 1000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/30/2020 4 NDC:75525-001-20 2000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/30/2020 5 NDC:75525-001-25 250 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/30/2020 6 NDC:75525-001-12 120 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/30/2020 7 NDC:75525-001-07 60 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/30/2020 8 NDC:75525-001-51 500 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/30/2020 9 NDC:75525-001-11 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/30/2020 10 NDC:75525-001-21 2000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/30/2020 11 NDC:75525-001-40 3875 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/30/2020 Labeler - IWANNA CORP SAS (883223177) Registrant - MASTER CUSTOMS BROKER (054631555) Establishment Name Address ID/FEI Business Operations IWANNA CORP SAS 883223177 manufacture(75525-001)