HAND SANITIZER
This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (70%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (1.00% v/v).
- Phenoxyethanol and chlorphenesin (0.3% v/v).
- Sterile distilled water or boiled cold water.
Indications and Usage
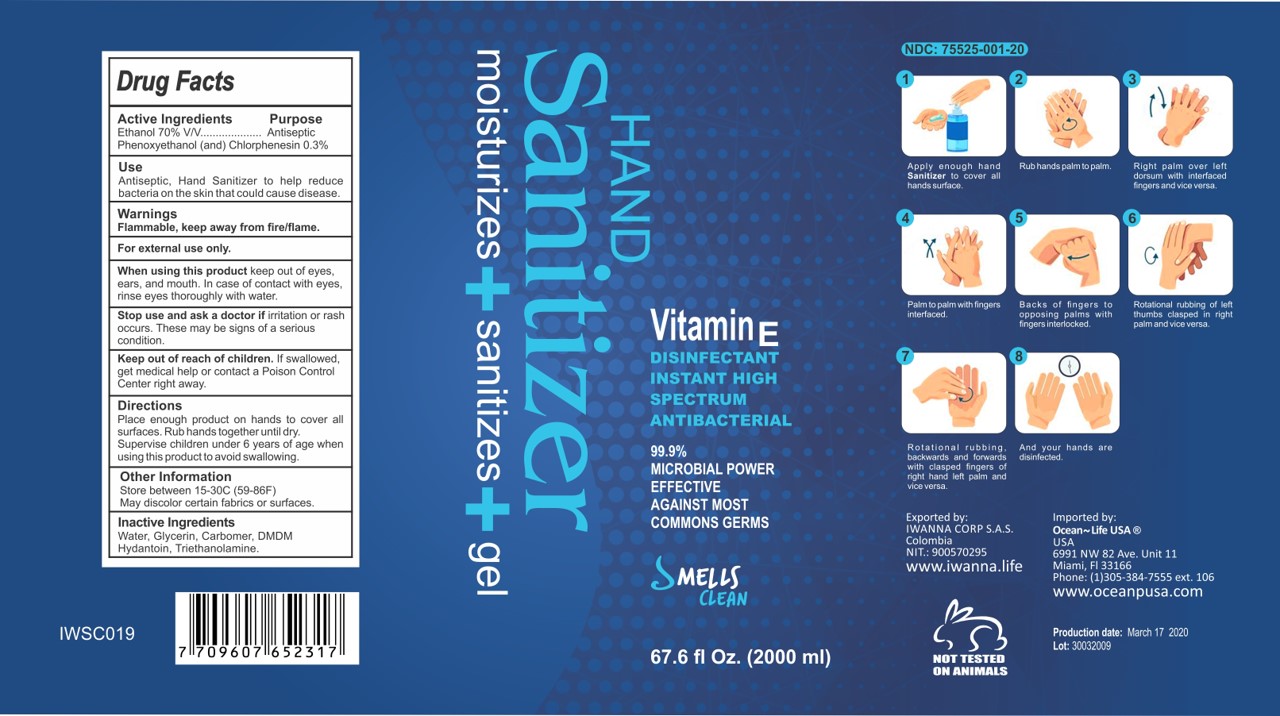
Apply enough hand Sanitizer to cover all hands surface
Rub hands palm to palm
Right palm over left dorsum with interfaced fingers and vice versa.
Palm to palm with fingers interfaced.
Backs of fingers to opposing palms with fingers interlocked.
Rotational rubbing of left thumbs clasped in right palmed and vice versa.
Rotational rubbing backwards and forwards with clasped fingers of right hand and left palm and vice versa.
And your hands are disinfected.
Warnings
Flammable. Keep away from fire or flame
For external use only.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Package Label - Principal Display Panel
Hand Sanitizer
moisturizes+sanitizes+gel
Hand Sanitizer- 500 ML
Hand Sanitizer
moisturizes+sanitizes+gel
16.9 fl. oz (500 mL) NDC: 75525-001-50

HAND SANITIZER 1000 ML
Hand Sanitizer
moisturizes+sanitizes+gel
33.8 fl. oz (1000 mL) NDC: 75525-001-10

HAND SANITIZER 2000 ML
Hand Sanitizer
moisturizes+sanitizes+gel
67.6 fl. oz (2000 mL) NDC: 75525-001-20
HAND SANITIZER 120 ML
Hand Sanitizer
moisturizes+sanitizes+gel
4.0 fl. oz (120 mL) NDC: 75525-001-12

HAND SANITIZER 250 ML
Hand Sanitizer
moisturizes+sanitizes+gel
8.4 fl. oz (250 mL) NDC: 75525-001-25

HAND SANITIZER 60 ML-DISPENSER
Hand Sanitizer
moisturizes+sanitizes+gel
2.02 FL OZ (60 ML)
NDC:75525-001-07

HAND SANITIZER 2000 ML DISP
Hand Sanitizer
moisturizes+sanitizes+gel
67.6 fl. oz (2000 mL) NDC: 75525-001-21

HAND SANITIZER 3875 ML DISP
Hand Sanitizer
moisturizes+sanitizes+gel
131 fl. oz (3875 mL) NDC: 75525-001-40



